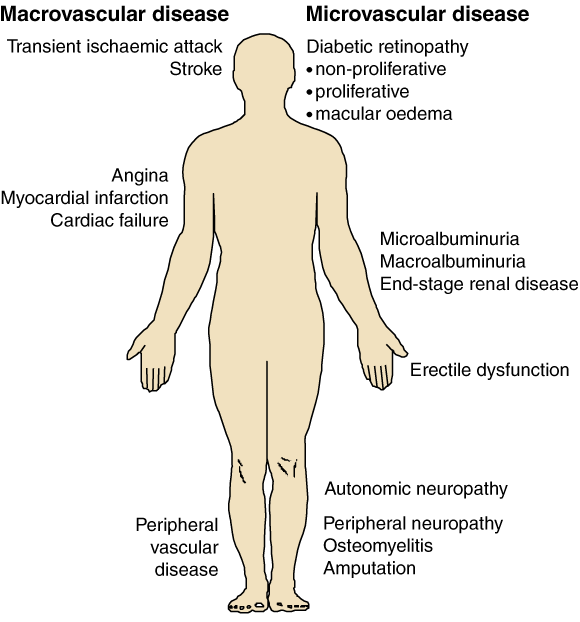
In Australia, 7% of the adult population has diabetes,1 and the number of affected individuals is rising. Complications are the major cause of associated morbidity and mortality. They are classified as macrovascular (affecting large arteries) or microvascular (affecting capillaries and small blood vessels) (Box 1). The presence of complications almost triples the mean cost of managing diabetes per patient, from $4000 to $10 000 per annum.2 The major aim of diabetes management is to prevent complications.
Among people with diabetes, about 15% have type 1 (formerly known as insulin-dependent diabetes), while about 85% have type 2 (formerly known as non-insulin-dependent diabetes).
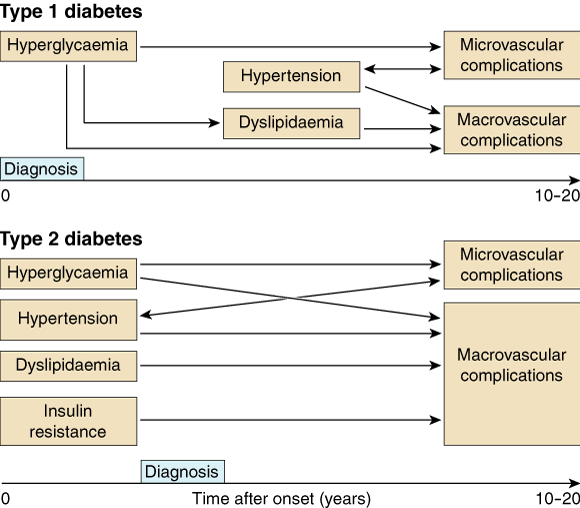
In type 1 diabetes, the major risk is microvascular complications, although macrovascular complications are also increased. The primary risk factor is hyperglycaemia, although other risk factors, such as hypertension and dyslipidaemia, may occur secondary to uncontrolled hyperglycaemia or renal disease (Box 2). Complications are therefore usually acquired after diagnosis.
In contrast, type 2 diabetes is usually part of the “metabolic syndrome”, which is associated with other risk factors from early in the disease process, including abdominal obesity, hypertension, dyslipidaemia, a prothrombotic state and insulin resistance (Box 2). Although macrovascular disease is the major cause of morbidity and mortality in type 2 diabetes,3 microvascular complications are often present when diabetes is diagnosed, even in people with no symptoms. Prevalences at diagnosis are: retinopathy, about 20%; neuropathy, 9%; and overt diabetic nephropathy, up to 10%. Type 2 diabetes increases the risk of coronary heart disease two- to fourfold and abolishes the protectiveness of female sex observed in the non-diabetic population.4 The presence of diabetes also worsens the prognosis of coronary heart disease.
Large-vessel disease, including coronary heart disease and stroke, is the greatest overall cause of morbidity and mortality in diabetes. Preventing these complications in type 2 diabetes, which is often associated with other cardiovascular risk factors, is a major challenge.
Finding strategies to reduce the development of macrovascular complications has been challenging. The United Kingdom Prospective Diabetes Study (UKPDS), to date the largest and longest prospective randomised trial in people with type 2 diabetes, showed that intensive blood glucose control alone failed to reduce macrovascular complications significantly,5,6 although it did reduce microvascular complications. However, the same trial showed that treating hypertension did reduce macrovascular complications.
Since the UKPDS, the approach has broadened, with other trials confirming the benefit of treating hypertension6-8 and showing significant benefits from treating dyslipidaemia.9-12 In major trials of lipid-lowering therapy, diabetic subgroups appeared to benefit more than those without diabetes.9,12 The recent publication of a trial of a multifactorial approach to prevent cardiovascular disease in people with type 2 diabetes suggests that the greatest benefits are seen when glucose, blood pressure and lipid levels are targeted simultaneously.3
Dietary modification, regular exercise and smoking cessation are recommended to help prevent cardiovascular disease, based on evidence from short-term lifestyle-intervention studies and observational studies.13 Lifestyle change also halves the progression from impaired glucose tolerance to type 2 diabetes.14 As complications are often present in type 2 diabetes at diagnosis, prevention has an important role in reducing complications, as does early detection (discussed previously in this series15).
Lifestyle measures may also reduce other cardiovascular risk factors. Moderation of salt intake, weight reduction and exercise reduce blood pressure. Regular exercise also lowers cholesterol levels, and may be more effective than a low-cholesterol diet.16
The optimal diet to reduce cardiovascular risk is unclear. Traditionally, a low-fat diet has been recommended, but this is being challenged by observational studies that a “Mediterranean-style” diet, emphasising vegetables, monounsaturated fats and whole grains, may better prevent cardiovascular events.17 The recent emphasis on carbohydrates with a low glycaemic index (ie, slowly absorbed) has not been examined in long-term interventional studies, but reduction in levels of glycosylated haemoglobin (HbA1c) has been achieved.18
In practice, lifestyle changes are difficult to achieve. A multifactorial intervention study found no significant difference in smoking status, exercise history or body mass index between a group that received intensive education about the benefits of exercise and smoking cessation and a control group.3
Fifty per cent of people with type 2 diabetes have hypertension (blood pressure > 140/90 mmHg),19 and systolic hypertension is the main disorder in the elderly. In type 1 diabetes, hypertension is less common but remains an important modifiable risk factor.
In people with diabetes, as blood pressure levels increase there is a parallel increase in cardiovascular disease, diabetic retinopathy and nephropathy.19 Randomised controlled trials have demonstrated clear benefits of lowering blood pressure. In the UKPDS trial, “tight” blood pressure control with angiotensin-converting enzyme (ACE) inhibitors or β-blockers significantly reduced diabetes-related events and diabetes-related deaths.6 The benefit of treating hypertension exceeded the benefit of treating hyperglycaemia.
Other trials have found that lower blood pressure targets further reduce macrovascular complications. For example, the Hypertension Optimal Treatment (HOT) Study found that the optimal diastolic blood pressure was 82.6 mmHg.7 Trials have been unable to define an optimal lower limit of blood pressure, as lower pressure is associated with lower risk even within the “normal” range.19 Consequently, the recommended blood pressure target for individuals with diabetes has been falling in recent years and is now ≤ 130/80 mmHg.19
Is it important how blood pressure is lowered? Although some trials have shown agent-specific effects, the consensus of a large number of hypertension trials is that the major effect is mediated through the reduction in blood pressure itself. Some classes of antihypertensives (ACE inhibitors, β-blockers and diuretics) have been repeatedly shown to reduce cardiovascular events in patients with essential hypertension, making them “first-line” agents for treating hypertension. In diabetes, ACE inhibitors and angiotensin II receptor antagonists are considered “first-line” agents for treating hypertension because of their roles in preventing and treating diabetic nephropathy (see below). Individual antihypertensives may also be selected because of their effects in specific situations, such as β-blockers after myocardial infarction, and ACE inhibitors and diuretics in congestive cardiac failure. Most patients require three or more drugs to reach target blood pressure.
The characteristic lipid abnormality in patients with type 2 diabetes is atherogenic dyslipidaemia — raised levels of both triglycerides and LDL cholesterol, and a low level of high-density lipoprotein (HDL) cholesterol. Statins (HMG-CoA [3-hydroxy-3-methylglutaryl coenzyme A] reductase inhibitors) have small effects on triglycerides and only modestly increase HDL cholesterol levels. However, secondary prevention trials in coronary heart disease, such as the Scandinavian Simvastatin Survival Study and the CARE trial,9 suggest that statins benefit diabetic patients at least as much as non-diabetic patients. Two recently published trials, the Heart Protection Study (HPS)10 and the Anglo–Scandinavian Cardiac Outcomes Trial (ASCOT),11 showed that individuals with vascular disease and those in a high-risk primary-prevention group benefited from statin treatment, even if cholesterol levels were relatively normal. It is important to note that such an aggressive approach to lipid-lowering is not currently reflected in current Pharmaceutical Benefits Scheme guidelines.
Fibrates such as gemfibrozil are potentially superior to statins in treating diabetic dyslipidaemia, as they increase HDL cholesterol and lower triglyceride level. However, large trials comparable to the statin trials are lacking. The results of the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study, examining the effect of feno-fibrate, are awaited with interest.
Combining a statin with gemfibrozil may be necessary in cases of severe combined dyslipidaemia. As this increases the risk of myopathy and hepatic dysfunction, monitoring of creatine kinase and liver function is advisable.
The UKPDS showed that intensive control of hyperglycaemia with sulfonylurea or insulin did not significantly reduce the risk of myocardial infarction or stroke (P = 0.05). However, subgroup analysis of 342 obese patients suggested that metformin therapy reduced the risk of myocardial infarction.20 Thus, metformin is the drug of first choice in overweight patients with type 2 diabetes.
The failure of the UKPDS to demonstrate a benefit of a sulfonlyurea or insulin on macrovascular complications may reflect the study design or the limitations of the drugs themselves. Although sulfonylurea and insulin initially reduce HbA1c level, neither prevents a subsequent inexorable rise over time, reflecting a progressive decline in pancreatic function. The new thiazolidinediones (“glitazones”) target insulin resistance and reduce HbA1c level by about 1 percentage point, similar to the initial reduction seen with sulfonylureas or metformin, but maintain the reduction for up to 4 years. Their effect on macrovascular complications is unknown.
Low-dose aspirin therapy is recommended for all people with diabetes and another risk factor, such as hypertension.7,21
A recent Danish study found that macrovascular complications in patients at high risk can be reduced through a multifactorial approach (involving behaviour modification and pharmacological therapy targeting hyperglycaemia, hypertension, dyslipidaemia and microalbuminuria, plus low-dose aspirin).3 This treatment was associated with an impressive reduction in cardiovascular events, equivalent to a number needed to treat for 5 years of 3.2, as well as significant reductions in diabetic nephropathy and retinopathy. There is no way of knowing which component of the “package” of interventions provided the greatest benefit, emphasising the need for a multifactorial approach to treating type 2 diabetes and the metabolic syndrome.
The Danish trial also revealed the limitations of current therapies (Box 3).3 Blood glucose targets were achieved in only 15% of patients, and smoking cessation advice was ineffective in both groups. Lipid and blood pressure targets were achieved by more than half of the intensive multifactorial treatment group. Even greater benefits may be possible if newer treatments could achieve the targets more often.
The major mechanism of microvascular disease is the toxic effect of prolonged hyperglycaemia (Box 2), with hypertension a further exacerbating factor. Microvascular complications seldom occur in isolation. Screening for microvascular disease enables intervention at the earliest possible stage, maximising the effectiveness of treatment (Box 4). Data from trials over the past 10 years show that controlling hyperglycaemia and hypertension reduces microvascular complications in both type 1 and type 2 diabetes.
Diabetic nephropathy is the most common cause of end-stage renal disease (ESRD), accounting for 40% of new cases in Western countries. About 20%–30% of patients with diabetes have evidence of overt diabetic nephropathy, defined as persistent clinically detectable proteinuria in association with hypertension and reduced glomerular filtration rate.22 Aboriginal Australians, New Zealand Mäori and South Pacific Islanders have particularly high rates of nephropathy and are at very high risk of progression to ESRD.
The earliest sign of diabetic renal disease is the presence of subclinical increases in urinary albumin excretion, termed microalbuminuria (urinary albumin excretion rate, 30–300 mg/24 h or 20–200 μg/min; or albumin–creatinine ratio > 2.5 mg/mmol in men and > 3.5 mg/mmol in women). Annual screening of people with diabetes for this complication is recommended. Microalbuminuria identifies individuals at high risk of progressing to macroalbuminuria (albumin excretion ≥ 300 mg/24 h, equivalent to total protein excretion ≥ 0.5 g/24 h), and also at risk of developing ESRD over a period of 10–20 years. Microalbuminuria is also an independent risk factor for cardiovascular disease.
Diabetic retinopathy is the leading cause of blindness in the adult population.23 In type 1 diabetes, almost all patients develop signs of retinopathy in the first 20 years. In type 2 diabetes, up to a third of patients have retinopathy at diagnosis,24 increasing to two-thirds within 20 years.
In the earliest stages of diabetic retinopathy, the characteristic abnormality is increased vascular permeability. Without treatment, microvascular occlusions occur, resulting in retinal ischaemia and, eventually, the growth of new vessels, termed proliferative retinopathy. Macular oedema, caused by increased vascular permeability, may occur at any stage.
The longer the duration of diabetes, the greater the risk of diabetic retinopathy. The most important treatable risk factors are hyperglycaemia and hypertension. Screening for diabetic retinopathy should be undertaken at least once every 2 years, and preferably annually, to allow early identification of treatable disease. Otherwise, diabetic retinopathy progresses silently until visual loss occurs.
Retinopathy is treated with laser photocoagulation, which usually prevents further loss but generally does not restore vision. For proliferative and severe non-proliferative retin-opathy, pan-retinal laser photocoagulation is used. The greatest benefit is in patients with high-risk changes (new vessels on the optic disc or vitreous haemorrhage). Clinically significant macular oedema is treated with focal laser photocoagulation therapy.
The Diabetes Control and Complications Trial25 and the UKPDS5 established the importance of intensive blood-glucose control in reducing the risk of microvascular complications (target HbA1c level ≤ 7%). For both diabetic retinopathy and nephropathy, the benefit of good glycaemic control appears to be greatest in the early stages. It has not been so clearly demonstrated that glycaemic control delays the progression of overt nephropathy, and intensified glucose control may temporarily exacerbate proliferative retinopathy.
Treatment of hypertension reduces the development and progression of microvascular complications in diabetes. Most of the supporting evidence comes from trials of the effects of inhibitors of the renin–angiotensin system on diabetic nephropathy. Drugs inhibiting this system, such as ACE inhibitors and angiotensin II receptor antagonists, generally have a greater benefit than other classes of anti-hypertensive drugs.
ACE inhibitors reduce the progression of microalbumin-uria to macroalbuminuria in patients with type 1 diabetes, and the decline in glomerular filtration rate in those with macroalbuminuria.26,27 ACE inhibitors also reduce micro-vascular complications in patients with type 2 diabetes and hypertension.6 More recently, trials have shown that angio-tensin II receptor antagonists reduce the rate of progression of microalbuminuria to macroalbuminuria and the rate of decline in glomerular filtration rate in overt nephropathy in patients with type 2 diabetes and hypertension (see case report, Box 5).28-30
Although the weight of trial data supports the use of ACE inhibitors in both types of diabetes, and angiotensin II receptor antagonists in type 2 diabetes, large “head-to-head” trials have not been conducted. Small trials suggest that the combination of an ACE inhibitor and angiotensin II receptor antagonist may have an additional renoprotective effect.31
The presence of diabetes should prompt aggressive management of cardiovascular risk factors, particularly hypertension and dyslipidaemia, to prevent macrovascular disease, as intensive management of these risk factors is of greater benefit for people with diabetes than for those without diabetes. Clinical trials in type 1 and type 2 diabetes over the past decade have shown that aggressive management of hyperglycaemia and hypertension significantly reduces the risk of developing diabetic nephropathy, retinopathy and peripheral neuropathy. ACE inhibitors and low-dose aspirin are indicated in people with diabetes and other cardiovascular risk factors (see case report, Box 5).
3: Benefit and ease of modifying risk factors for macrovascular and microvascular disease
|
|
Benefit |
|||||||||
Risk factor |
Ease of modification5 |
Macrovascular disease |
Microvascular disease |
||||||||
Blood glucose |
Relatively hard to reduce Target reached (HbA1c level < 6.5%) in < 20% in long-term therapy |
Benefit marginal in randomised controlled trials,3 except with metformin in overweight people16 |
Significant benefits3,16,18 |
||||||||
Blood pressure |
Target reached (systolic BP < 130 mmHg, diastolic BP < 80 mmHg) in 50%–60% Multiple agents often required |
Significant benefits from even modest reduction4,11,26 |
Significant benefits from even modest reduction4,26 Additional benefits for nephropathy with renin–angiotensin system inhibitors19-24 |
||||||||
Smoking |
Difficult |
Probable benefit |
Probable benefit |
||||||||
Dyslipidaemia |
Targets reached (total cholesterol < 4.53 mmol/L; triglycerides < 1.69 mmol/L) in 30%–50% |
Significant benefits13-15,25 |
Benefits unclear |
||||||||
HbA1c = glycosylated haemoglobin. BP = blood pressure. |
|||||||||||
4: Screening for complications of diabetes
Complication |
When to start |
Frequency |
How to screen |
||||||||
Macrovascular disease |
|
Annually |
Ask about symptoms (intermittent claudication, angina [may be atypical] and transient ischaemic attack/stroke) Examine pedal pulses, auscultate for bruits, and Use a low “threshold” for electrocardiography and exercise stress testing |
||||||||
Microvascular disease |
|
|
|||||||||
Retinopathy |
Every 1–2 years |
Dilated fundus ophthalmoscopy (usually by an ophthalmologist or optometrist) or Fundal photography |
|||||||||
Nephropathy |
Annually |
Random spot urine sample for albumin–creatinine ratio* or Albumin excretion rate in 24 h or timed overnight urine collection† |
|||||||||
Peripheral neuropathy |
Annually |
Assess protective sensation in feet (eg, with Semmes–Weinstein 10 g monofilament17) Look for evidence of maldistribution of pressure (eg, calluses), and Assess vascular supply and skin integrity |
|||||||||
Autonomic neuropathy |
Annually |
Ask about symptoms (nausea and vomiting, nocturnal diarrhoea, postural hypotension, erectile dysfunction) |
|||||||||
* Reference range for albumin–creatinine ratio, < 2.5 mg/mmol in men, < 3.5 mg/mmol in women. † Microalbuminuria should be confirmed with at least 2 specimens, preferably by measurement of albumin excretion rate. False positive results occur with recent exercise, urinary tract infection, fever, marked hypertension, marked hyperglycaemia, congestive cardiac failure and haematuria. |
|||||||||||
5: Case report — deteriorating type 2 diabetes
Presentation: A 62-year-old woman with type 2 diabetes presented to her general practitioner with a recent rise in glycosylated haemogolobin (HbA1c) level. Diabetes had been diagnosed 3 years previously. She had attended an education program covering diet, exercise, self-monitoring of blood glucose and foot care. She did not smoke. With a diet and exercise plan, her HbA1c level was maintained in the range 6.0%–6.5% for over 2 years, indicating satisfactory glycaemic control. However, over the previous 6 months, despite her continuing to follow dietary recommendations reasonably well and walking most days for over half an hour, her HbA1c level rose to 8.0%, indicating poor glycaemic control.
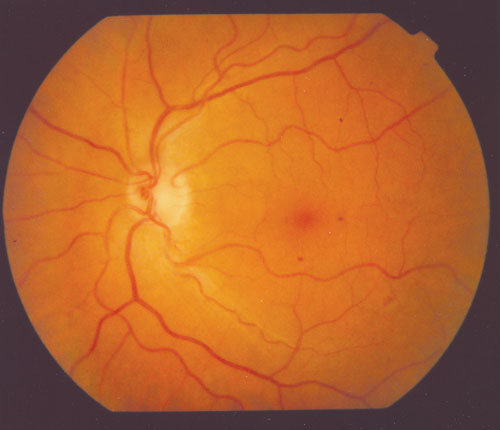
Retinal photograph showing microaneurysms at 1, 3 and 6 o’clock relative to the fovea.
Examination and investigations: Her blood pressure was 140/90 mmHg. Cholesterol levels were: total, 5.6 mmol/L (reference range [RR], < 5.5 mmol/L); high-density lipoprotein (HDL), 0.9 mmol/L (RR, 1.0–2.0 mmol/L); and triglycerides, 2.7 mmol/L (RR, < 2.0 mmol/L). Body mass index of 28.3 kg/m2 (RR, < 25 kg/m2) was in the overweight range. Eye examination showed a few microaneurysms. Her serum creatinine level was 0.10 mmol/L (RR, 0.03–0.11 mmol/L), and urinary albumin excretion rate was in the microalbuminuric range at 100 μg/min (RR < 20 μg/min). She had no signs of peripheral neuropathy.
Management: She was encouraged to walk for half an hour at least every second day and to take increased care with her diet.
After six weeks, she had lost 1 kg, but her blood glucose level remained raised. A repeat urine test confirmed microalbuminuria.
As she was overweight and had normal renal function, metformin was the most appropriate initial treatment. The dose was titrated from 250 mg twice daily to 1000 mg twice daily over the next few weeks.
Several weeks later, her blood glucose levels had greatly improved, and she had not developed the side effects of nausea, bloating or diarrhoea.
3 months later, her HbA1c level had decreased to 6.8%, but her blood pressure remained raised at 142/95 mmHg.
Four months later, her blood pressure had fallen to 135/88 mmHg. Serum creatinine level remained unchanged, and she did not develop hyperkalaemia.
The irbesartan dose was increased to 300 mg/day.
Six months later, urinary albumin excretion rate had fallen to 62 μg/min, and blood pressure to 130/85 mmHg.
A thiazide diuretic, hydrochlorothiazide (12.5 mg/day), was added to the regimen.
She was also advised to take simvastatin (40 mg/day), after it was explained that only a small number of people like her (10–15) would need to be treated for 5 years to prevent one cardiovascular event. She was warned to report myalgia, which may be a side effect.
As her major risk for diabetes complications was from cardiovascular disease (risk factors, diabetes, hypertension, dyslipidaemia, obesity and microalbuminuria), aspirin (150 mg/day) was also added to the regimen.
- 1. Dunstan D, Zimmet P, Welborn T, et al. The rising prevalence of diabetes and impaired glucose tolerance: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care 2002; 25: 829-834.
- 2. Colagiuri S, Colagiuri R, Conway B. DiabCost Australia. Assessing the burden of Type 2 diabetes in Australia 2002. Available at: http://www.diabetesnsw.com.au/docs/pdfs/2003109113947.pdf(accessed Oct 2003).
- 3. Gaede P, Vedal P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383-393.
- 4. Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham Study. Diabetes Care 1979; 2: 120-126.
- 5. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837-853.
- 6. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998; 317: 703-713.
- 7. Hansson L, Zanchetti A, Westerling S, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351: 1755-1762.
- 8. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000; 355: 253-259.
- 9. Sacks FM, Pfeffer MA, Braunwald E, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996; 335: 1001-1009.
- 10. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360: 7-22.
- 11. Sever PS, Dahlof B, Ostergren J, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361: 1149-1158.
- 12. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease : the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344: 1383-1389.
- 13. Hu F, Stampfer M, Solomon C. Physical activity and risk for cardiovascular events in diabetic women. Ann Intern Med 2001; 134: 96-105.
- 14. Tuomilehto J, Lindstrom J, Uusitupa M, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343-1350.
- 15. Shaw JE, Chisholm DJ. 1: Epidemiology and prevention of type 2 diabetes and the metabolic syndrome. Med J Aust 2003; 179: 379-383. <eMJA full text>
- 16. Stefanick ML, Mackey S, Wood PD, et al. Effects of diet and exercise in men and postmenopausal women with low levels of HDL cholesterol and high levels of LDL cholesterol. N Engl J Med 1998; 339: 12-20.
- 17. Trichopoulou A, Costacou T, Trichopoulos D, et al. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003; 348: 2599-2608.
- 18. Gilbertson HR, Brand-Miller JC, Werther GA, et al. The effect of flexible low glycemic index dietary advice versus measured carbohydrate exchange diets on glycemic control in children with type 1 diabetes. Diabetes Care 2001; 24: 1137-1143.
- 19. National Institutes of Health. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7). Available at: www.nhlbi.nih.gov/guidelines/hypertension (accessed Oct 2003).
- 20. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 854-865.
- 21. Standards of medical care for patients with diabetes mellitus — American Diabetes Association. Diabetes Care 2003; 26 Suppl 1: S33-S50.
- 22. Marshall SM. Clinical features and management of diabetic nephropathy. In: Pickup JC, Williams G, editors. Textbook of diabetes. Boston: Blackwell Publishing, 2003.
- 23. VanNewkirk MR, Weih L, Taylor HR, et al. Cause-specific prevalence of bilateral visual impairment in Victoria, Australia: the Visual Impairment Project. Ophthalmology 2001; 108: 960-967.
- 24. Fong DS, Aiello L, Gardner TW, et al. Diabetic retinopathy. Diabetes Care 2003; 26 Suppl 1: S99-S102.
- 25. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993; 329: 977-986.
- 26. Lewis EJ, Hunsicker LG, Rohde RD, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329: 1456-1462.
- 27. Laffel LM, McGill JB, Gans DJ. The beneficial effect of angiotensin-converting enzyme inhibition with captopril on diabetic nephropathy in normotensive IDDM patients with microalbuminuria. North American Microalbuminuria Study Group. Am J Med 1995; 99: 497-504.
- 28. Parving HH, Lehnert H, Brochner-Mortensen J, et al. Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001; 345: 870-878.
- 29. Brenner BM, Cooper ME, de Zeeuw D, et al, for the RENAAL Study Investigators (Brigham and Women's Hospital, Boston; University of Melbourne, Victoria, Australia; Hennepin County Medical Center, Minneapolis). Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861-869.
- 30. Lewis EJ, Hunsicker LG, Raz I, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851-860.
- 31. Rossing K, Christensen PK, Parving HH, et al. Dual blockade of the renin-angiotensin system in diabetic nephropathy : a randomized double-blind cross-over study. Diabetes Care 2002; 25: 95-100.





Abstract
Diabetes complications are common and almost triple the annual cost of managing diabetes.
Microvascular complications are the major risk in type 1 diabetes, while macrovascular complications are the major cause of morbidity and mortality in type 2 diabetes.
Control of hyperglycaemia (target HbA1c level ≤ 7%) and hypertension (target blood pressure ≤ 130/80 mmHg) prevents microvascular complications in both types of diabetes; a multifactorial approach, comprising behaviour modification and pharmacological therapy for all risk factors, reduces the development of micro- and macrovascular complications in type 2 diabetes.
The benefit of treating dyslipidaemia is at least as great in the diabetic population as in the non-diabetic population.
Angiotensin-converting enzyme inhibitors and low-dose aspirin are indicated in people with diabetes and other cardiovascular risk factors.
Regular annual screening for diabetes complications allows treatable disease to be identified.