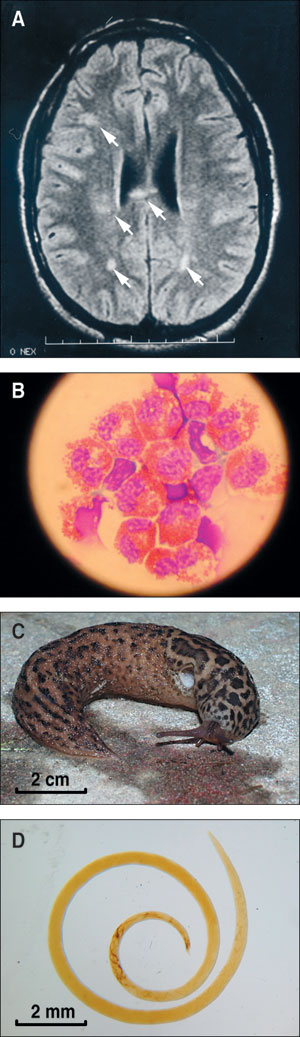
Clinical record
In 2001, a young man was admitted to hospital with a 3-day history of gradual-onset headache, nausea, vomiting, neck stiffness and photophobia. Three weeks earlier he had experienced a gastrointestinal illness (nausea, abdominal cramps, diarrhoea, myalgia and fever) that persisted for 1 week. On examination, he had a low-grade fever and meningism. A cerebral computed tomography scan showed no abnormality. Peripheral blood eosinophilia (1.6 x 109/L; reference range [RR], < 0.44 x 109/L) was noted, and examination of cerebrospinal fluid (CSF) showed 530 x 106/L monocytes (RR, < 5 x 106/L), 22 x 106/L red cells (RR, < 1 x 106/L), no eosinophils on routine staining, a raised protein level of 1.07 g/L (RR, < 0.45 g/L) and a normal glucose level. He was treated with intravenous aciclovir for 6 days. A CSF polymerase chain reaction test for herpes simplex virus-1 (HSV-1) and HSV-2 subsequently gave negative results. Serological tests for Strongyloides and Angiostrongylus were negative. CSF and blood cultures showed no growth. Twelve days after admission, he was discharged from hospital with resolving meningism. Five days later, increasing headache and drowsiness prompted his admission to another hospital. He was afebrile, drowsy and irritable, with gross bilateral papilloedema. He described mild paraesthesiae in both hands. He had peripheral blood eosinophilia (3.1 x 109/L). Magnetic resonance imaging of the brain with gadolinium contrast showed multiple focal enhancing lesions in the deep white matter of both cerebral hemispheres, including the corpus callosum (Figure A). CSF from cisternal puncture was cloudy, under high pressure and, on routine toluidine blue staining, had 1008 x 106/L polymorphonuclear cells (RR, < 5 x 106/L), 186 x 106/L monocytes and 21 x 106/L red cells. Further staining to detect eosinophils was requested, and 90% of the polymorphonuclear cells were found to be eosinophils (Figure B). His CSF protein level was elevated at 0.8 g/L and glucose level 2.6 mmol/L (50% serum glucose). India ink, Ziehl–Neelsen and Gram stains were negative. Repeated questioning revealed that the patient had ingested, 5 weeks earlier, for a dare, two slugs from a garden in a Sydney suburb. Repeat Angiostrongylus immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA), tested in parallel with the first specimen, was positive, confirming seroconversion. Several leopard slugs, Limax maximus (Figure C), taken from the Sydney garden were dissected without finding larvae (Figure D), but no rats from the vicinity were examined for this infection. Treatment comprised measures to reduce intracranial pressure with repeated CSF drainage, acetazolamide and dexamethasone, initially given intravenously, and then orally. CSF drainage consisted of one cisterna magna puncture and two lumbar punctures. Specific anthelmintic agents were not given. No ocular larvae were seen on regular formal ophthalmological review. He improved gradually and, after 17 days in hospital, was discharged with instructions to take a reducing dose of dexamethasone over 4 weeks. His final lumbar puncture 1 month after admission showed an almost normal CSF protein level (0.5 g/L) and a reduction in CSF white cell count (107 x 106/L; 8% eosinophils). After 5 months, he successfully returned to full-time studies and competitive sport. |
B: Spun-down cerebrospinal fluid cells (Romanowsky stain: original magnification x400). C: Limax maximus, the leopard slug, an intermediate host for Angiostrongylus cantonensis. D: Adult female Angiostrongylus cantonensis from the lungs of Rattus norvegicus. |
This is the first reported case of human eosinophilic meningitis due to Angiostrongylus cantonensis acquired in Sydney. The first A. cantonensis infection in humans reported in Australia was from Brisbane in 1971.1 More recently, a fatal case occurred in a child who ingested molluscs in a suburban Brisbane garden.2 Over the past 10 years, Angiostrongylus has been isolated from dogs, flying foxes, marsupials and zoo primates in Sydney.3
Angiostrongylus cantonensis, also known as Parastrongylus cantonensis, is the commonest infectious cause of eosinophilic meningitis worldwide and is endemic in South-East Asia and the Pacific Basin.4 The other, rarer parasitic causes of eosinophilic meningitis are not endemic to Australia.5 Non-infectious causes of eosinophilic meningitis include haematological malignancies, antibiotics (ciprofloxacin, intraventricular gentamicin or vancomycin) and idiopathic hypereosinophilic syndrome.6
The lifecycle of the parasite from the adult stage in the definitive rat host through the intermediate mollusc host has been described previously.2 Humans become accidental hosts when they ingest the larval stage in raw or undercooked molluscs or crustaceans or in fresh vegetables contaminated by infected molluscs.5 The diagnosis of angiostrongyliasis in a patient with acute eosinophilic meningoencephalitis is supported by a history of mollusc ingestion, but eliciting this may require specific questioning.
Symptoms occur 2–45 days after ingestion.7 The most common symptom is headache. Paraesthesiae are frequently reported.6 In our patient, the acute febrile gastrointestinal illness 6 days after consuming the slugs may have been caused by invasion of the parasite through the intestinal wall. Initial entry into the meninges, and subsequent migration through brain parenchyma, caused the clinical picture of meningitis followed by encephalitis. Seizures or other focal neurological symptoms may occur. Peripheral blood and CSF eosinophilia strongly support a diagnosis of Angiostrongylus meningoencephalitis, but may appear only later in the course of the illness, or, in a minority of cases, not at all.4,7,8
It is important to emphasise that eosinophils may not easily be differentiated from neutrophils on routine microbiological staining, such as with toluidine blue wet films. In aseptic meningitis, particularly associated with peripheral eosinophilia, specific Romanowsky stains, such as May–Grünwald–Giemsa or Wright stains, should be performed. An ELISA measuring total IgG can be diagnostic. The ELISA used to demonstrate seroconversion in this case utilised somatic antigens from adult A. cantonensis. The ELISA can be performed on serum or CSF. The Institute for Clinical Pathology and Medical Research at Westmead Hospital is the only centre in New South Wales performing the assay. As parasitologically proven cases are rare, it is difficult to determine the sensitivity and specificity of this assay.
Angiostrongylus meningitis is usually mild and resolves spontaneously over 6 weeks. Occasionally, cases are severe and may have chronic sequelae.5,6,8 No randomised controlled studies have assessed optimal management, but repeated CSF drainage may give symptomatic relief.8,9 Steroid treatment appears to be beneficial, presumably reducing CSF pressure and inflammatory response. Regimens reportedly of benefit include prednisolone 30–60 mg/day for 5 days,8 prednisolone 40–60 mg/day with weaning over a few weeks,9 and prednisolone 60 mg/day for 2 weeks.10 The use of anthelmintic agents is controversial. Generally, avoidance of anthelmintic agents has been recommended on the (theoretical) basis of their potential for harm owing to the inflammatory response provoked by antigen release after parasite death.4,8
A. cantonensis should be considered as a cause of aseptic meningitis in patients with paraesthesiae and peripheral eosinophilia, and a history of exposure to undercooked molluscs or crustaceans. This report highlights the wider distribution of this parasite in Australia and, in particular, its close proximity to urban populations.
- Don S Pryor1
- Pam Konecny2
- Sanjaya N Senanayake3
- John Walker4
- 1 University of New South Wales, St George Hospital, Kogarah, NSW.
- 2 Public Health Unit, Prince of Wales Hospital Campus, Randwick, NSW.
- 3 University of Sydney, Centre for Infectious Diseases and Microbiology, Westmead Hospital, Sydney, NSW.
- 1. Gutteridge BH, Bhaibulaya M, Findlater C. Human larval meningitis possibly following lettuce ingestion in Brisbane. Pathology 1972; 4: 63-64.
- 2. Prociv P. Parasitic meningitis: crossing paths with the rat lungworm (Angiostrongylus cantonensis). Med J Aust 1999; 170: 517-518.
- 3. Prociv P, Carlisle MS. The spread of Angiostrongylus cantonensis in Australia. Southeast Asian J Trop Med Public Health 2001; 32 Suppl 2: 126-128.
- 4. Slom TJ, Cortese MM, Gerber SI, et al. An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. N Engl J Med 2002; 346: 668-675.
- 5. Hughes AJ, Biggs BA. Parasitic worms of the central nervous system: an Australian perspective. Intern Med J 2002; 32: 541-553.
- 6. Weller PF. Eosinophilic meningitis. Am J Med 1993; 95: 250-253.
- 7. Hwang KP, Chen ER. Clinical studies on Angiostrongyliasis cantonensis among children in Taiwan. Southeast Asian J Trop Med Pub Health 1991; 22 (Suppl): 194-199.
- 8. Punyagupta S, Juttijudata P, Bunnag T. Eosinophilic meningitis in Thailand: clinical studies of 484 typical cases probably caused by Angiostrongylus cantonensis. Am J Trop Med Hyg 1975; 24: 921-931.
- 9. Pien FD, Pien BC. Angiostrongylus cantonensis eosinophilic meningitis. Int J Infect Dis 1999; 3: 161-163.
- 10. Chotmongkol V, Sawanyawisuth K, Thavornpitak Y. Corticosteroid treatment of eosinophilic meningitis. Clin Infect Dis 2000; 31: 660-662.