Chronic suppurative otitis media (CSOM) is a disease of poverty. It is very common among Australian Aboriginal children, with the prevalence exceeding the World Health Organization’s definition of a “massive” public health problem.1,2 CSOM is a chronic infection of the middle ear, defined as otorrhoea of at least 2 weeks’ duration in the presence of tympanic membrane (TM) perforation.1 Research in 1998–2001 showed that 23% of Aboriginal children from remote regions of the Northern Territory had evidence of perforated tympanic membranes.3 In Aboriginal children, CSOM usually commences in infancy, is evident within a few weeks of birth,4 causes conductive and sensorineural hearing loss,5,6 and has adverse effects on child development.7 It is recurrent,8 can persist into adulthood,9 may result in a need for hospitalisation10 and may have other neurological and life-threatening sequelae.11
Ototopical antibiotic treatment with aminoglycosides is more effective than systemic antibiotic therapy in eliminating otorrhoea in CSOM, while dry mopping alone appears to be ineffective.12 A topical aminoglycoside antibiotic containing framycetin (0.5%), gramicidin, and dexamethasone (FGD) is the mainstay of treatment for CSOM in Australia,13 even though trials have reported that topical fluoroquinolones (such as ciprofloxacin) are more effective at drying up otorrhoea in CSOM than topical aminoglycosides.12
Although clinical studies report no changes in hearing levels when topical aminoglycosides are used to treat CSOM,12 they have been shown to be ototoxic in animal studies,14 and are used purposely to cause vestibular toxicity in patients with Menières disease.15 By contrast, topical fluoroquinolones are non-toxic when administered into the middle ear,16-18 and there is negligible systemic absorption in children.19
Here we report the first Aboriginal community-controlled, multicentre, double-blind, randomised controlled trial among Aboriginal children in Australia (known as the NACCHO [National Aboriginal Community Controlled Health Organisation] Ear Trial) to compare the effectiveness of topical ciprofloxacin (CIP) and FGD for treating CSOM in Aboriginal children.
The trial was conducted in eight Aboriginal communities in northern Western Australia and Queensland through Aboriginal Community Controlled Health Services (ACCHS). The Aboriginal community-controlled research process has been described elsewhere (unpublished data).
Children aged less than 15 years with at least 2 weeks of otorrhoea and TM perforation were eligible for inclusion.1 Exclusion criteria were: current febrile illness, current antibiotic use or use in the preceding 2 weeks, allergy to ototopical medications and specific allergy to fluoroquinolones, need for renal dialysis, recent ear surgery or an in-situ grommet or tympanostomy tube, mastoid surgery in the preceding 12 months, congenital ear or hearing problems, obstructed middle ear (eg, polyp), pregnancy, and being unlikely to be resident in the study region over the follow-up period.
Informed consent was obtained from each child’s parents.
A statistical program20 was used to produce balanced random sequences for each site to assign the two ototopical medications to a list of client identification numbers. Participants were then assigned a client number according to the sequence, which was concealed from them and from investigators for the duration of the trial and analysis. None of the investigators, health team nor the children knew which agents had been allocated (double-blind).
Both topical antibiotics had the same pH (4.5), colour, viscosity and similar storage requirements, but they had slightly different odours. To achieve blinding, third parties transferred the agents into brown glass bottles (each containing 8 mL) with droppers, checked them for sterility, labelled them with client numbers, generic product safety information, and site number, packaged them according to the randomisation sequence and distributed them to clinic sites.
Children with bilateral CSOM received treatment for both ears, although only one ear (randomly chosen) was monitored for the study.
Children received either ciprofloxacin (0.3%, Ciloxan, Alcon Labs Pty Ltd) or framycetin (0.5%), gramicidin and dexamethasone (Sofradex, Aventis Pharma Pty Ltd) — five drops twice daily for 9 days.
A clinical cure was defined as a complete absence of discharge in the middle ear and canal determined by otoscopy. During the treatment period, each child was assessed daily, with half the ototopical treatments applied by health workers and the other half by parents/guardians. If clinical cure was achieved by Day 10, treatment was stopped and the child was reassessed at Day 14. Guardians were instructed in applying eardrops (supine into external meatus), including the use of tragal pressure (pressing several times on the flap of skin in front of the ear canal) which aids middle-ear penetration.21
Ears were cleaned twice each day before the eardrops were administered. Cleaning comprised gentle syringing of the discharge in the external ear canal with 0.5% povidone-iodine solution until the TM perforation was clear of purulent material. Children were not permitted to swim while receiving ototopical medications.
All recruitment, treatment, and clinical assessment was conducted by trained Aboriginal Health Workers (AHWs) at each participating ACCHS. Most workers had previously completed the Commonwealth-sponsored training program by Australian Hearing.22 Acquisition of skills in otoscopy, video otoscopy/photography capture, and audiometry during training were audited by Australian Hearing. All sites had calibrated screening audiometers (Welch Allyn, Skaneateles Falls, NY), soundproof rooms, and otoscopy and video otoscopy equipment.
Baseline measurements included: age, sex, height, weight, living conditions, family size, type of housing, prior history and treatment of otorrhoea, and risk factors for CSOM. The clinical assessment at baseline and at Days 10 and 14 included otoscopy for otorrhoea, grading of the discharge, sample of the discharge for bacteriological examinations, size estimates of the TM perforation, pure-tone audiometry (air conduction) for hearing thresholds at frequencies of 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz (after ear cleaning and drying with tissue paper spears). Pure-tone audiometry was conducted in sound-proof rooms in children aged over 3 years.
An ear swab was obtained by inserting a sterile swab deep in the ear canal before commencing ear cleaning. This method was selected because pathogens isolated from the middle ear correlate well with those from the external canal in patients with CSOM-related otorrhoea.23 All microbiological processing of swabs was blinded and was conducted at the same site in Perth to assure uniform standards. Queensland sites used an interstate courier transport system, while Western Australian sites used existing storage and transport policies (insulated packing with ice bricks), to ensure swabs would reach the laboratory within 72 hours of sampling.
Ear swabs were cultured on cysteine lactose electrolyte deficient agar, blood agar, and horse haematin agar (all incubated at 35°C for 48 hours in 5% CO2), as well as blood agar containing colistin and nalidixic acid and Sabourauds agar (incubated at 35°C and 30°C, respectively, for 48 hours in air). All isolates of conventional pathogens were reported individually. Other organisms not usually colonising the healthy external ear canal were reported only when seen as a heavy predominant growth. Susceptibility testing of significant isolates was performed according to NCCLS guidelines.
The size of TM perforation was assessed by visual grading as a proportion of the TM surface area. A sample of digital images of tympanic membranes (using video otoscopy) were copied to CD-ROM and sent to two otolaryngologists for validation of perforation size as reported by AHWs.
Adherence to treatment was assessed by the number of AHW checks per child and the proportion of scheduled drops applied by the AHW and guardian.
We hypothesised that the use of ciprofloxacin in children would lead to a 20 percentage point increase in resolved CSOM after 9–10 days of twice-daily treatment than would the use of FGD. From cure percentages in previous randomised trials of ototopical aminoglycosides, we calculated that 100 children were needed in each treatment arm to detect an improvement in resolution of CSOM from 50% to 70% with a power of 80% at an α level of 5%.24,25 To allow for a 30% loss to follow-up, 300 children were needed (30–60 per recruitment site).
Univariate statistics: Categorical variables were described as percentages. As the distributions of most of the numerical variables proved to be skewed, medians were used as measures of central tendency, and 25% and 75% percentiles as measures of dispersion for descriptions of numerical information. The hearing threshold level for each child was averaged over four frequencies. Differences in average hearing threshold and grade of perforation were calculated for each child to compare pretreatment and posttreatment outcomes.
Bivariate statistics: Comparisons between treatment groups were performed using exact versions of χ2-type tests for nominal and exact-trend tests for ordinal information. Numerical variables were compared using exact versions of the Mann–Whitney tests. For all statistical tests, exact P values are stated; values below 0.05 were regarded as significant. Confidence limits (95%) for proportions were calculated using exact binomial probabilities.
Multivariable statistics: Stepwise logistic regression modelling for dichotomous outcomes was applied to check for potential confounders of the bivariately observed difference in rates of successful cures between the two treatment regimens. No “intention to treat” analysis was conducted as no information on children lost to follow-up was available. No “interim” analysis was performed.
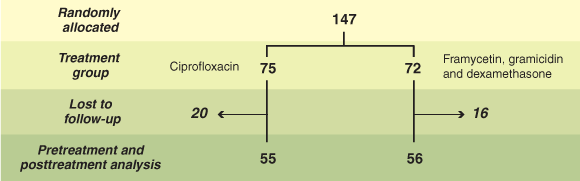
Between 1 April 2001 and 30 June 2002, 147 children commenced study treatment (Box 1). The strict inclusion criteria resulted in slower recruitment than predicted, and the trial was stopped because of resource constraints before achieving the intended sample size.
Thirty-six children randomly allocated to the treatment arms did not complete the follow-up schedule: 18 were lost to follow-up (CIP, 10; FGD, 8), 8 had incomplete follow-up (CIP, 3; FGD, 5), nine withdrew for reasons unrelated to the study, mainly because of family leaving (CIP, 6; FGD, 3), and one (CIP) withdrew as otorrhoea cleared up before the scheduled assessment. The final sample for primary endpoint analysis comprised 111 children aged 1–14 years (see Box 1). The follow-up period after starting treatment ranged from 10 to 21 days.
Overall, cure of CSOM was observed in 71 of the 111 children (64%). Cure was significantly correlated with the type of treatment (P = 0.009). In the FGD group, 29 of the 56 children (51.8%) were cured compared with 42 of the 55 (76.4%) in the CIP group. This reflects an absolute difference of 24.6% (95% CI, 15.8%–33.4%). No significant difference (before and after treatment) was found in the size of TM perforation or the level of hearing impairment, nor was there a difference in these variables according to the ototopical medication used (Box 2).
We found no association between clinical cure and any of the assessed potential confounders (age, sex, history of exposure to cigarette smoke inside dwellings, crowding in households, other children with CSOM in the household, duration and grade of discharge at baseline, history of prior ear infection, history of swimming with head underwater in the preceding 2 weeks, and number of visits by AHWs).
Children who were not cured after a course of ototopical medication (31/83 with baseline audiometry) were more likely to be those with poor hearing at baseline (average hearing threshold, 36.2 dB compared with 23.7 dB, P = 0.01). No relationship between clinical cure and the initial size of the TM perforation was shown.
We found ciprofloxacin (0.3%) ear drops were 47% more likely to cure CSOM in Aboriginal children after a single course of twice-daily treatment than combined framycetin (0.5%), gramicidin and dexamethasone ear drops.
None of the potential confounders assessed, which may have otherwise explained the difference in clinical cure, were of any relevance. We found no differences between the two antibiotic treatment groups in concordance with the study protocol, which supports the success of blinding. Whether the antiseptic effect of ear cleaning contributed overall to clinical cure remains unknown, as a control arm without antibiotics was excluded for ethical reasons.
The proportion of children cured with ciprofloxacin (76%) is consistent with that reported in children from hospital-based studies (69%–91%).26,27 These studies used ototopical ciprofloxacin two or three times daily for 10–14 days (a total of 18–20 mg) compared with our 9-day schedule (total 13.5 mg, or 6% of a single 250 mg tablet). To make blinding possible in our study, the children in the FDG group received 10 drops per day instead of the recommended 12 drops.13 We believe this slightly lower dose is unlikely to have altered the outcome. CSOM cure fractions of 30%–87% with topical aminoglycosides have been reported.27,28
No change in hearing or TM healing was evident through the use of ototopicals within the follow-up period (a longer term analysis is in progress). Resolution of otorrhoea does not immediately resolve conductive hearing loss, because of persistent TM perforation and disruption of the ossicular chain. However, if CSOM-related otorrhoea is cured, surgery for persistent TM perforations can improve hearing by an average of 13 dB.12
We found no evidence that the use of ototopical ciprofloxacin led to antibiotic resistance in baseline or subsequent pathogens isolated from the ear. The environment of the middle ear is not conducive to the development of resistance to ciprofloxacin, as topical doses ensure an antibiotic concentration thousands of times higher than the minimum inhibitory concentration for pathogens, while minimising systemic exposure.29
Many of the Aboriginal children in our study had air-conduction hearing thresholds indicating enough hearing impairment to warrant hearing aids (WHO criteria, ≥ 26 dB).30 Over a third of the children also had impaired hearing in the other ear, although only 13% had bilateral CSOM. The children in our study also had well recognised risk factors for CSOM, such as serious overcrowding, high proportions of CSOM among siblings and contacts, and significant passive exposure to cigarette smoke. While only 0.5% of the general Australian population had seven or more people resident in 3-bedroom dwellings in 1996,31 this was the norm for 54% of our children. The proportion passively exposed to cigarette smoke (68.5%) is also much higher than smoking prevalence among parents of infants in the general population (28.9%).32 These data underscore the need to address economic, social and environmental inequities faced by the Aboriginal population.
The use of aminoglycosides in patients with a perforated TM is fraught with medicolegal and ethical implications, as product information precludes such use.33 Ignoring these issues when ototopical fluoroquinolones have replaced aminoglycosides as treatment for CSOM in the United States, Japan, Spain and other countries,34 is unjustifiable. Australian federal authorities should look at increasing access to twice-daily ototopical ciprofloxacin or other fluoroquinolones as first-line treatment for CSOM in Aboriginal children. Special access schemes may be required and should be considered urgently.
2: Outcome variables and difference from baseline in the ear monitored for the trial
Post-treatment variables |
All (n = 111) |
CIP (n = 55) |
FGD (n = 56) |
P |
|||||||
Number with clinical cure
|
71 |
42 |
29 |
0.009 |
|||||||
Grade of tympanic membrane perforation [n (%)] |
(n = 69) |
(n = 36) |
(n = 33) |
0.18 |
|||||||
Nil |
2 (2.9) |
1 (2.8) |
1 (3.0) |
|
|||||||
Small (0–25%) |
45 (65.2) |
24 (66.7) |
21 (63.6) |
|
|||||||
Medium (25%–50%) |
9 (13) |
8 (22.2) |
1 (3.0) |
|
|||||||
Large (50%–75%) |
11 (15.9) |
3 (8.3) |
8 (24.2) |
|
|||||||
Very large (> 75%) |
2 (2.9) |
0 |
2 (6.1) |
|
|||||||
Difference in grade of TM perforation [n (%)] |
(n = 64) |
(n = 34) |
(n = 30) |
0.21 |
|||||||
Larger (1-step increment) |
10 (15.6) |
3 (8.8) |
7 (23.3) |
|
|||||||
No change |
38 (59.4) |
22 (64.7) |
16 (53.3) |
|
|||||||
Smaller (1-step decrement) |
14 (21.9) |
7 (20.6) |
7 (23.3) |
|
|||||||
Smaller (2-step decrement) |
2 (3.1) |
2 (5.9) |
0 |
|
|||||||
Average hearing threshold [median (IQR)]† |
(n = 52) |
(n = 25) |
(n = 27) |
0.59 |
|||||||
|
23.1 dB (15.0–36.3 dB) |
28.8 dB (14.3–37.5 dB) |
22.5 dB (15.0–35.0 dB) |
|
|||||||
Difference in average hearing threshold [median (IQR)]† |
(n = 49) |
(n = 23) |
(n = 26) |
0.62 |
|||||||
|
1.3 dB (-5.0–11.9 dB) |
1.3 dB (-7.5–12.5 dB) |
1.9 dB (-2.5–11.8 dB) |
|
|||||||
Level of hearing impairment [n (%)]† |
(n = 52) |
(n = 25) |
(n = 27) |
0.57 |
|||||||
25 dB or better |
27 (51.9) |
48.0 (12) |
55.6 (15) |
|
|||||||
26–40 dB |
18 (34.6) |
36.0 (9) |
33.3 (9) |
|
|||||||
41–60 dB |
7 (13.5) |
4 (16.0) |
3 (11.1) |
|
|||||||
61–80 dB |
0 |
0 |
0 |
|
|||||||
Difference in level of hearing impairment [n (%)]† |
(n = 49) |
(n = 23) |
(n = 26) |
0.99 |
|||||||
Worse (1-step increment) |
11 (22.4) |
26.1 (6) |
19.2 (5) |
|
|||||||
No change |
26 (53.1) |
47.8 (11) |
57.7 (15) |
|
|||||||
Improved (1-step decrement) |
10 (20.4) |
21.7 (5) |
19.2 (5) |
|
|||||||
Improved (2-step decrement) |
2 (4.1) |
4.3 (1) |
3.8 (1) |
|
|||||||
CIP = ciprofloxacin. FGD = framycetin (0.5%), gramicidin, and dexamethasone. IQR = interquartile range. dB = decibels. * Defined as a complete absence of discharge in the middle ear and canal determined by otoscopy in the ear monitored for the trial. † Air conduction averaged for 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz (an improved difference in level of hearing impairment means there was a decrease in the average hearing threshold). |
|||||||||||
Received 7 April 2003, accepted 1 July 2003
- Sophie Couzos1
- Traven Lea2
- Margaret Culbong3
- Reinhold Mueller4
- Richard Murray5
- 1 National Aboriginal Community Controlled Health Organisation, Deakin, ACT.
- 2 School of Public Health and Tropical Medicine, James Cook University, Townsville, QLD.
- 3 Kimberley Aboriginal Medical Services Council, Broome, WA.
NACCHO acknowledges its former Chair, the late Dr Puggy Hunter as the instigator of the trial and thanks its Board of Directors for direction and oversight, and the following Aboriginal Community Controlled Health Services: Geraldton Regional Aboriginal Medical Service, Wirraka Maya Health Service, Kimberley Aboriginal Medical Services Council (Bidyadanga Community Clinic, Yura Yungi Aboriginal Medical Service, Derby Aboriginal Medical Service), Aboriginal and Islander Community Health Service, Brisbane, Goondir Aboriginal and Torres Strait Islander Community Health Service, Townsville Aboriginal and Islander Community Health Service, Cherbourg Health Action Group. Thanks also to the technical consortium for advice on the protocol and training: James Cook University (Professor Ian Ring), Telethon Institute for Child Health Research (Dr Deborah Lehmann, Dr Sandra Eades, Dr Anne Read), Otolaryngologists (Mr Harvey Coates, Mr Francis Lannigan), WA Centre for Pathology and Medical Research (Dr Clay Golledge, Mr Rod Bowman), Australian Hearing (Mr Ian Henderson) and Irene Nannup and the Derbarl Yerrigan Aboriginal Medical Service, Perth WA. The study was supported by: National Health and Medical Research Council, the Commonwealth Department for Education, Training and Youth Affairs, and RioTinto Aboriginal Foundation. Alcon Laboratories Australia Pty Ltd provided the pharmaceuticals.
None identified.
- 1. WHO/CIBA Foundation Workshop. Prevention of hearing impairment from chronic otitis media. London, 19–21 November 1996. Geneva: World Health Organization, 1998. (WHO/PDH/98.4). Available at: www.who.int/pbd/pdh/Docs/COM-Cover-sum.html (accessed Jan 2003).
- 2. Couzos S, Metcalf S, Murray R, for the National Aboriginal Community Controlled Health Organisation. Systematic review of existing evidence and primary care guidelines on the management of otitis media in Aboriginal and Torres Strait Islander populations. Canberra: Commonwealth Department of Health and Family Services, Office for Aboriginal and Torres Strait Islander Health Services, 2001. Available at: www.health.gov.au/oatsih/pubs/omp.htm (accessed Jan 2003).
- 3. Mackerras DE, Reid A, Sayers SM, et al. Growth and morbidity in children in the Aboriginal Birth Cohort Study: the urban-remote differential. Med J Aust 2003; 178: 56-60. <MJA full text>
- 4. Boswell J, Nienhuys T. Onset of otitis media in the first eight weeks of life in Aboriginal and non-Aboriginal Australian infants. Ann Otol Rhinol Laryngol 1995; 104: 542-549.
- 5. Paparella MM, Morizono T, Le CT, et al. Sensorineural hearing loss in otitis media. Ann Otol Rhinol Laryngol 1984; 93: 623-629.
- 6. El-Sayed Y. Bone conduction impairment in uncomplicated chronic suppurative otitis media. Am J Otolaryngol 1998; 19: 149-153.
- 7. Lewis N. Otitis media and linguistic incompetence. Arch Otolaryngol 1976; 102: 387-390.
- 8. Boswell JB, Niehuys TG. Patterns of persistent otitis media in the first year of life in Aboriginal and non-Aboriginal infants. Ann Otol Rhinol Laryngol 1996; 105: 893-900.
- 9. Hoy W, Norman RJ, Hayhurst BG, Pugsley DJ. A health profile of adults in a Northern Territory Aboriginal community, with an emphasis on preventable morbidities. Aust N Z J Public Health 1997; 21: 121–126.
- 10. Mak D, MacKendrick A, Weeks S, Plant AJ. Middle-ear disease in remote Aboriginal Australia: a field assessment of surgical outcomes. J Laryngol Otol 2000; 114: 26-32.
- 11. Kangsanarak J, Fooanant S, Ruckphaopunt K, et al. Extracranial and intracranial complications of suppurative otitis media. Report of 102 cases. J Laryngol Otol 1993; 107: 999-1004.
- 12. Acuin J, Smith A, Mackenzie I. Interventions for chronic suppurative otitis media (Cochrane review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
- 13. Therapeutic guidelines: antibiotic. 12th ed. Melbourne: Therapeutic Guidelines Limited, 2003.
- 14. Rohn GN, Meyerhoff WL, Wright CG. Ototoxicity of topical agents. Otolaryngol Clin North Am 1993; 26: 747-758.
- 15. Rutka J. Update on topical ototoxicity in chronic suppurative otitis media. Ear Nose Throat J 2002; 81 (8 Suppl 1): 18-19.
- 16. Barlow DW, Duckert LG, Kreig CS, Gates GA. Ototoxicity of topical otomicrobial agents. Acta Otolaryngol (Stockh) 1995; 115: 231-235.
- 17. Brownlee RE, Hulka GF, Prazma J, Pillsbury HC 3rd. Ciprofloxacin. Use as a topical otic preparation. Arch Otolaryngol Head Neck Surg 1992; 118: 392-396.
- 18. Gates GA. Safety of ofloxacin otic and other ototopical treatments in animal models and in humans. Pediatr Infect Dis J 2001; 20: 104-107, 120-122.
- 19. Force RW, Hart MC, Plummer SA, et al. Topical ciprofloxacin for otorrhoea after tympanostomy tube placement. Arch Otolaryngol Head Neck Surg 1995; 121: 880-884.
- 20. SPSS [computer program]. Version 10. Chicago, Ill: SPSS Inc, 2000.
- 21. Hebert RL 2nd, Vick ML, King GE, Bent JP 3rd. Tympanostomy tubes and otic suspensions: do they reach the middle ear space? Otolaryngol Head Neck Surg 2000; 122: 330-333.
- 22. Commonwealth Office of Aboriginal and Torres Strait Islander Health. The report on Commonwealth funded hearing services to Aboriginal and Torres Strait Islander people: strategies for future action. Available at: www.health.gov.au/oatsih/pubs/hearingreport.htm (accessed Jan 2003).
- 23. Wintermeyer SM, Nahata MC. Chronic suppurative otitis media. Ann Pharmacother 1994; 28: 1089-1099.
- 24. Smith A, Hatcher J, Mackenzie I, et al. Randomised controlled trial of treatment of chronic suppurative otitis media in Kenyan schoolchildren. Lancet 1996; 348: 1128-1133.
- 25. Eason RJ, Harding E, Nicholson R, et al. Chronic suppurative otitis media in the Solomon Islands: a prospective, microbiological, audiometric and therapeutic survey. N Z Med J 1986; 99: 812-815.
- 26. Wintermeyer SM, Hart MC, Nahata MC. Efficacy of ototopical ciprofloxacin in pediatric patients with otorrhea. Otolaryngol Head Neck Surg 1997; 116: 450-453.
- 27. Miro N, and the Spanish ENT Study Group. Controlled multicenter study on chronic suppurative otitis media treated with topical applications of ciprofloxacin 0.2% solution in single-dose containers or combination of polymyxin B, neomycin, and hydrocortisone suspension. Otolaryngol Head Neck Surg 2000; 123: 617-623.
- 28. Tutkun A, Ozagar A, Koc A, et al. Treatment of chronic ear disease. Topical ciprofloxacin vs topical gentamicin. Arch Otolaryngol Head Neck Surg 1995; 121: 1414-1416.
- 29. Klein JO. Strategies for decreasing multidrug antibiotic resistance: role of ototopical agents for treatment of middle ear infections. Am J Manag Care 2002; 8 (14 Suppl): S345-S352.
- 30. World Health Organization. Report of the first informal consultation on future programme developments for the prevention of deafness and hearing impairment (WHO/PDH/97.3). Geneva: WHO, 1997. Available at: www.who.int/pbd/pdh/Docs/Documents-Available-List-English.html (accessed Jan 2003).
- 31. Australian Bureau of Statistics. Housing and infrastructure in Aboriginal and Torres Strait Islander Communities. Canberra: ABS, 1999. (Catalogue No 4710.0).
- 32. Turrell G, Battistutta D, McGuffog I. Social determinants of smoking among parents with infants. Aust N Z J Public Health 2002; 26: 30-37.
- 33. Aventis Pharma. Product information for Sofradex ear drops and ointment. Available at: wnfsyd.appco.com.au/APPCO_WEB/onlineindex.htm (accessed Mar 2003).
- 34. Ghosh S, Panarese A, Parker AJ, Bull PD. Quinolone ear drops for chronic otitis media. They are safer and more effective than aminoglycosides. BMJ 2000; 321: 126-127.





Abstract
Objectives: To compare the effectiveness of ototopical ciprofloxacin (0.3%; CIP) with framycetin (0.5%), gramicidin, dexamethasone (FGD) eardrops (5 drops twice daily for 9 days) together with povidone-iodine (0.5%) ear cleaning as treatments for chronic suppurative otitis media (CSOM) in Aboriginal children.
Design and participants: Aboriginal community-controlled, community-based, multicentre, double-blind, randomised controlled trial in eight Aboriginal Community Controlled Health Services across northern Australia, involving 147 Aboriginal children with CSOM.
Main outcome measures: Resolution of otorrhoea (clinical cure), proportion of children with healed perforated tympanic membrane (TM) and improved hearing, 10–21 days after starting treatment.
Results: 111 children aged 1–14 years (CIP, 55; FGD, 56) completed treatment. CSOM cures occurred in 64% (CIP, 76.4%; FGD, 51.8%), with a significantly higher rate in the ciprofloxacin group (P = 0.009, absolute difference of 24.6% [95% CI, 15.8%–33.4%]). TM perforation size and the level of hearing impairment did not change. Pseudomonas aeruginosa was the most common bacterial pathogen (in 47.6%), while respiratory pathogens were rare (in 5.7%).
Conclusions: Twice-daily ear cleaning and topical ciprofloxacin is effective at community-level in achieving cure for CSOM. Healthcare providers to Aboriginal children with CSOM should be given special access to provide ototopical ciprofloxacin as first-line treatment.