Assays of the cardiac-specific troponins T and I (cTnT and cTnI) have rapidly become the criterion standard for chemical diagnosis of myocardial damage since they were introduced in the 1990s. These assays are more sensitive and specific than assays of the creatine kinase-MB isoenzyme (CK-MB), which they have largely replaced.1
Interpreting results of troponin assays is easy if levels are at the upper end of the abnormal range, but presents a challenge at the lower end. "Acute coronary syndrome" has been devised as a category for patients with troponin levels of indeterminate significance pending results of further investigations.2
Troponin challenges doctors because its behaviour is similar but not identical to that of CK-MB. Levels of troponin, like CK-MB, do not rise immediately after myocardial damage but take about 6 hours to become diagnostically reliable. However, the kinetics of disappearance of the two substances from the bloodstream differ markedly. CK-MB is rapidly released into the blood in the few hours after infarction, reaches a peak concentration quickly, and then declines with a half-life of about 12 hours. In contrast, troponins continue to be shed into the bloodstream while the damaged myocardium is undergoing repair and are continuously cleared. Consequently, a steady state level of troponin is achieved rapidly after an infarct and often maintained for up to a week, before falling back to pre-infarct levels over little more than a day.3
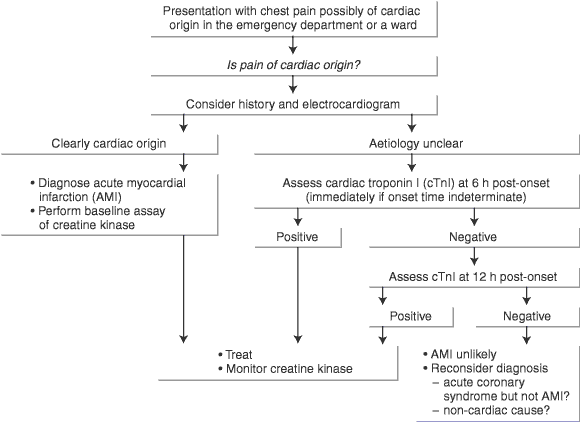
The diagnostic dilemma is thus clear: although a troponin assay is the new criterion standard for diagnosing a myocardial infarct, it cannot be used either to follow progress after an infarct or, within the week thereafter, to corroborate a clinically suspected extension of the original infarct or to diagnose a new infarct. Nevertheless, authoritative protocols suggest that a troponin level measured at 6 hours after symptom onset, and, if this is negative, again at 12 hours, safely determines whether a patient's symptoms are caused by an acute myocardial infarction (AMI).4,5
Is the troponin assay used appropriately in the diagnostic process when chest pain is encountered? Is it overused? This report describes the introduction of a troponin assay and its subsequent use in three Melbourne hospitals.
The study was undertaken in three hospitals in Melbourne, Victoria. Each had an independent emergency department and coronary care unit, but their pathology laboratories were under the supervision of a single chemical pathologist (myself). Hospital A is a 330-bed tertiary-care institution, while Hospitals B and C are suburban hospitals, with 300 and 290 beds, respectively. At the time of the study in May 2002, annual admissions of patients with an acute coronary syndrome (including AMI) at the three hospitals numbered about 900, 500 and 1800, respectively.
The troponin assay used was the AxSYM cTnI assay (Abbott Laboratories, Abbott Park, IL, USA). It was introduced at Hospital A in December 1998 and at Hospitals B and C in February 2000. Before introduction of the assay, I consulted with the directors of cardiology and the emergency departments at each hospital to devise a protocol for its use. This protocol was based on evidence and recommendations in the 1998 draft of the Standard of laboratory practice of the American National Academy of Clinical Biochemistry (NACB)6 (Box 1).
The protocol was issued jointly by myself and the director of cardiology of each hospital at the time the assay was introduced. It was distributed to hospital medical officers and nurse unit managers in all wards and the emergency department. We asked that it be prominently displayed in ward offices. The protocol was also made available to any doctor or nurse who subsequently requested a copy, but it was not repromulgated.
Medical scientists in each laboratory were instructed to screen all requests for a troponin assay and to reject those that were inappropriate. As pertinent clinical details (including time of symptom onset) were rarely provided with requests, all first requests for an assay were processed as if specimens were taken 6 hours after symptom onset. "Inappropriate" was defined as:
Specimens taken < 4 hours after a previous troponin assay; or
Requests within 7 days of a positive cTnI result.
Requests rejected by the laboratory generated one of two pre-scripted comments: "A troponin has been measured for this patient in recent hours. If chest pain is ongoing, a repeat troponin should not be done till at least 4 hours after the last one"; or "Troponin I remains elevated for about 7 days after myocardial damage and thus cannot be used serially to monitor patient progress; use CK for this purpose".
Positive assay results were defined according to the manufacturer's recommendations with:
The reference range that excludes myocardial damage defined as cTnI level < 0.5 μg/L (97th percentile for a myocardially healthy reference group); and
A cTnI level > 2 μg/L indicating an AMI.
These definitions meet the requirements of the NACB Standard of laboratory practice5 and were printed on all reports of results. Because of the coefficient of variation of the assay, repeat assays were allowed after results in the range 1.5–2.5 μg/L.
The survey of outcome included all patients for whom a cTnI assay was requested between 1 and 7 May 2002. This was 42 months after initial introduction of the troponin assay at Hospital A, and 27 months after introduction at Hospitals B and C.
Laboratory records were audited to determine whether requests were single or part of a series, times between serial requests, appropriateness and laboratory actions. A new symptom episode was deemed to have arisen when more than 24 hours elapsed after a previously negative cTnI result was reported, except where stated otherwise on the request form.
Statistical analysis was performed using the Stata computer program.7 As this was a clinical audit using and reporting de-identified data, approval by a human research ethics committee was not sought.
Troponin assays were ordered during 351 symptom episodes involving 333 patients (Box 2). A single troponin assay was ordered in 194 symptom episodes (55% of all symptom episodes; range across hospitals, 54%–56%; x2 for all differences, P = 0.71). Thirteen of these 194 single assays (7%) had results diagnostic of an AMI. Five of these 13 were routine tests performed 24 h after coronary artery grafting (four patients) or aortic aneurysm repair (one patient), rather than in response to symptoms.
Serial assays were ordered in 157 symptom episodes (45%). Ordering of these assays appeared to follow the recommended protocol closely at all three hospitals, with mean time between serial assays more than 6 hours.
Sixty-four requests for assays were judged inappropriate (10% of all requests; range, 7%–13%). Medical scientists rejected 31 of these (5% of all requests). More of the inappropriate requests were rejected at Hospital A (18/24) than at Hospitals B and C (4/12 and 9/28, respectively).
This study found that use of troponin assays was remarkably uniform across the three hospitals in both its good and its bad aspects. Adherence to protocol for serial troponin testing intervals was adequate, but single troponin assays were also used extensively (in more than half of symptom episodes) and probably inappropriately.
Recognised protocols suggest that, in patients presenting with symptoms and signs suggesting AMI, this diagnosis can be excluded by two negative troponin results over about 12 hours after presentation.4,5 However, 93% of the 194 single assay requests gave negative results and, as they were not followed by a second (later) assay, were used in a manner at odds with recognised protocols for excluding AMI. In these cases, doctors may have decided on clinical grounds that a second assay was not required. For example, they may have initially overestimated the probability of a myocardial event and revised that estimate as the patient's symptoms evolved.8
Nonetheless, if doctors exclude recent myocardial damage on the basis of a single negative troponin result, they run the risk of missing an evolving infarct. The specificity of a troponin result rises with the time elapsed since myocardial damage begins; in the earliest hours of an infarct, it is too low to safely rule out the diagnosis. It could be argued that if a patient's symptoms generate sufficient clinical suspicion to justify a troponin assay, then AMI cannot be excluded until a second negative troponin result is obtained after a further 6 hours.
On the other hand, requests for serial assays followed the recommended protocol closely at all three hospitals. Mean times between serial tests were at least 6 hours, as required by the protocol, and stretched up to 8 hours. This prolongation reflects the reality of practice in busy tertiary level hospitals.
Laboratory scientists varied in their policing of the protocol. Those at Hospitals B and C were less likely to reject inappropriate specimens than those at Hospital A. No explanation for this difference is obvious.
Conclusions from this audit are twofold. Firstly, a rational approach was uniformly seen when serial troponin assays were used to diagnose AMI. Our support strategy may have helped produce and sustain this outcome. Initially, the strategy provided information about how to use troponin assays, and delivered it with suitable authority at the time the test was introduced. This information was continually reinforced by comments on reports of results. Continued screening of requests to determine appropriateness is needed to provide this ongoing reinforcement.
A weakness of this study is that it did not assess doctors' practice in the absence of the support strategy, and therefore cannot assess the extent to which the strategy modified practice. However, it would have been clinically and ethically improper to have introduced the assay without a support strategy.
Secondly, the finding that a large proportion of requests for troponin assays were single requests is troublesome. This suggests that some clinicians regard a single troponin assay as a remedy for diagnostic dilemmas. However, this is not necessarily so. We need to determine how patients who have a single troponin assay fare, and to educate their doctors not to use the assay in this fashion.
2: Use of troponin assays per symptom episode at three metropolitan hospitals
|
Hospital A (n = 122) |
Hospital B (n = 97) |
Hospital C (n = 132) |
Total (n = 351) |
|||||||
Number of patients |
112 |
93 |
128 |
333 |
|||||||
Assays per symptom episode |
|
|
|
|
|||||||
1 |
68 (56%) |
52 (54%) |
74 (56%) |
194 (55%) |
|||||||
2 |
33 (27%) |
24 (25%) |
27 (20%) |
84 (24%) |
|||||||
3 |
19 (16%) |
16 (16%) |
25 (19%) |
60 (17%) |
|||||||
> 3 |
2 (2%) |
5 (5%) |
6 (5%) |
13 (4%) |
|||||||
Total > 1 |
54 (44%) |
45 (46%) |
58 (44%) |
157 (45%) |
|||||||
Hours from 1st to 2nd assay* |
|
|
|
|
|||||||
Mean (95% CI) |
6.5 (5.7–7.3) |
6.7 (5.6–7.8) |
8.0 (6.9–9.1) |
7.1 (6.5–7.6) |
|||||||
Median (interquartile range) |
6 (5–7) |
6 (4–7) |
7 (6–8) |
6 (5–8) |
|||||||
Hours from 2nd to 3rd assay* |
|
|
|
|
|||||||
Mean (95% CI) |
8.0 (6.4–9.6) |
7.3 (6.0–8.6) |
8.0 (6.8–9.2) |
7.8 (7.0–8.6) |
|||||||
Median (interquartile range) |
7 (5–9) |
6 (5–8) |
7 (7–9) |
7 (6–8) |
|||||||
Inappropriate requests |
24 (11%) |
12 (7%) |
28 (13%) |
64 (10%) |
|||||||
Too soon |
6 (3%) |
6 (3.5%) |
3 (2%) |
15 (2%) |
|||||||
Within 7 days of positive result |
18 (8%) |
6 (3.5%) |
25 (11%) |
49 (8%) |
|||||||
Rejected by scientists |
18 (8%) |
4 (2%) |
9 (4%) |
31 (5%) |
|||||||
* For multiple assays. |
|||||||||||
Received 29 November 2002, accepted 17 April 2003
- 1. Apple FS, Wu AHB. Myocardial infarction redefined: Role of cardiac troponin testing. Clin Chem 2001; 47: 377-379.
- 2. Panteghini M. Acute coronary syndrome. Biochemical strategies in the troponin era. Chest 2002; 122: 1428-1435.
- 3. Collinson PO, Boa FG, Gaze DC. Measurement of cardiac troponins. Ann Clin Biochem 2001; 38: 423-449.
- 4. Alpert JS, Thygesen K, Antman E, Bassand JP. The Joint European Society of Cardiology/American College of Cardiology Committee. Myocardial infarction redefined — a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000; 36: 959-969.
- 5. Wu AHB, Apple FS, Warshaw MM, editors. Recommendations for the use of cardiac markers in coronary artery disease. Washington: American Association for Clinical Chemistry Press, 1999.
- 6. Wu A, for the NACB Committee. Draft standard of laboratory practice: use of cardiac markers in coronary artery disease. Considered 1998 Aug 4 and 5, at the Annual Scientific Meeting of the American Association for Clinical Chemistry. Washington: AACC Press, 1998.
- 7. StataCorp. Stata Statistical Software: Release 6. College Station TX: Stata Press, 1999.
- 8. Pauker SG, Kassirer JP. The threshold approach to clinical decision making. N Engl J Med 1980; 302: 1109-1117.





Abstract
Objective: To audit the appropriateness of use of a troponin I assay in three hospitals.
Design: Cross-sectional survey of use of a troponin assay.
Setting: Three hospitals in Melbourne, Victoria, each with an emergency department and a coronary care unit.
Participants: Patients for whom a troponin I assay was requested between 1 and 7 May 2002, 27–42 months after introduction of the assay.
Interventions: User-focused dissemination of relevant information, including protocols for use, from opinion leaders when the assay was introduced; continuous reinforcement of information in pathology reports.
Main outcome measures: Adherence to protocol for assay use.
Results: Troponin assays were requested for 333 patients during 351 symptom episodes. A single assay was used in 194 symptom episodes (55%), and serial assays in 157 (45%); proportions were statistically indistinguishable across all three hospitals (χ2; P = 0.71). Of the 194 single assays, 13 (7%) diagnosed a myocardial infarction. Serial troponin testing in all three hospitals followed the suggested protocol, with mean time between serial assays being more than 6 hours at all hospitals.
Conclusions: Adherence to the protocol for serial troponin assay intervals was adequate, but single troponin assays were used extensively and probably inappropriately.