The recent debate on legislation for creating human embryonic stem-cell lines in Australia has been confounded by issues concerning the use of human fetal tissue derived from therapeutic termination of pregnancies.1 Embryonic stem cells are different from fetal tissues, the former being derived from the inner cell mass of spare fertilised eggs, and the latter from a termination of pregnancy. Obtaining and using both of these tissues are sensitive issues in the community, because of the sanctity of life and defining when it begins.
Human fetal tissue has been used both therapeutically and for biomedical research in Australia since 1980.2 How human fetal tissue is obtained and what it is used for in Australia was first extensively reported in 1993,3 but its use seems to have had little public impact. This became apparent during the stem-cell debate in 2002, when it was announced that human embryonic stem cells could now be grown on a feeder layer of human fetal cells, as compared with the more traditional mouse cells.4 Indeed, a major national newspaper reported the use of human fetal tissue in medical research as novel.5
Here we examine the use of human fetal tissue in Australia for biomedical research. We document how such tissues have been used since 1994, the advances being made as a result of their use, and the extent to which researchers share this tissue.
Human fetal tissue is a scarce resource. Almost all of the tissue used is obtained from institutions where second trimester therapeutic terminations are performed. The gestational age of the tissue used varies between 8 and 20 weeks’ gestation, but the age of most is 14–18 weeks. Organs are more readily identified during the second trimester.
The major distribution centre for such tissue to medical researchers in Australia is the Diabetes Transplant Unit (DTU) at the Prince of Wales Hospital, Sydney. The DTU organises collection of tissue from therapeutically terminated pregnancies, sorts it, and then distributes relevant tissues to researchers who have requested them. An additional centre at the University of Sydney obtained a small supply of tissue separately during 1996–2002. The information we present here is the combined data from the DTU and the Sydney University units. It was our understanding that this represented the state of practice in Australia, as requests for fetal tissue are received from other centres, both within New South Wales and in other states. However, immediately prior to publication, we became aware that there is a researcher in Newcastle who has obtained 70 specimens of human fetal tissue of median age 11 weeks (range 8–19 weeks) between September 2002 and June 2003.
The number of fetuses obtained annually by us between 1994 and 2002 varied considerably (Box 1). The number was greater in the mid 1990s than in subsequent years, with an average of 219 annually in the earlier period, compared with 77 annually in the later period. An average of three samples of tissue are used from each fetus. The number of samples distributed to researchers annually varied from 156 to 472 (Box 1). The most commonly used tissues were eye (23%), bone/cartilage (23%), brain/spinal cord (22%), kidney (15%) and pancreas (8%).
From 1994 to 2002, 19 separate biomedical researchers at 12 separate Australian institutions (four universities, six major teaching hospitals and two research institutes) used human fetal tissue in their research. The actual tissues used (detailed in Box 2) were determined by the individual research projects. In 1994, eye tissue made up nearly half of the distributed tissue. There was an increase in the use of bone/cartilage during 1996–2001, kidneys during 1996–1999, and liver and spinal cord over the past 4 years.
From 1994 to 2002, the tissue was used exclusively for biomedical research. In the previous decade, it was also used for therapeutic purposes,3 in an attempt to replenish ß cells in people with type 1 diabetes.6 These trials, which demonstrated survival but not function of the grafts, were discontinued as supply of second trimester tissue was limited. The research projects for which human fetal tissues were used included tissue development, mechanism of action of viruses, transplantation studies to treat insulin-dependent diabetes, complications of high glucose levels, drug testing, and the use of feeder cells for maintaining human embryonic stem cells in an undifferentiated state (Box 3). As this research could not have been done with tissue from animals, it provided valuable information that is specific to humans.
Comparative studies with normal human fetal tissue increase our understanding of the pathogenesis of a number of diseases. These include inflammatory disorders of the nervous system (eg, multiple sclerosis), disorders of the eye (eg, retinopathy of prematurity and optic nerve colobomas), and osteoarthritis. From these studies, novel treatment strategies are being developed. An example in multiple sclerosis treatment is the use of agents that stimulate the kynurenine pathway, which protects neurones from being attacked.7
A measure of the usefulness of human fetal tissue in medical research is the number of publications in peer reviewed journals that arise from it. A list of publications was obtained both directly (from researchers who receive the fetal tissue) and from reviewing PubMed. Over the past decade there were 74 publications, comprising eight articles on bones,8 eight on kidney,9 22 on central and peripheral nervous tissue,7,10 23 on eyes,11,12 five on pancreas,13 three on liver,14 two on placenta, and one each on adrenal gland, skin and heart.
In October 1983, the National Health and Medical Research Council introduced guidelines governing the use of fetal tissue for biomedical research in Australia.15 These guidelines ensure that (i) there is distinct separation between the patient and the research group in both the decision-making process and the proximity of the research to the clinical ward; and (ii) parental consent is obtained for the use of the tissue. These guidelines stipulate that the human fetal tissue used must be from terminations of pregnancy at less than 20 weeks’ gestation and where the weight of the fetus is less than 400 g. It is considered that a human fetus after this gestation period has a chance, albeit very remote, of surviving outside the uterus. The guidelines allow the use of tissue from the time of implantation in the uterus.
The Australian guidelines will change to some extent if the draft of a new document proposed by the Australian Health Ethics Committee in February this year is accepted in its current form.16 Tissue from fetuses at less than 8 weeks’ gestation will be regarded as embryos, and it seems that gaining consent to use such tissue will be much more onerous than it is at present. A licence may be required, as is currently the case for experimentation on spare fertilised eggs.
The perception of ethics changes with time. In the early 1980s, the consent form that was approved by institutional ethics committees to be signed by pregnant mothers allowing use of the fetal tissue consisted of two sentences. By the mid 1980s, ethics committees altered their requirements, and the form was expanded to provide a more in-depth explanation of what the tissue was used for. In the late 1990s, requirements changed again, with the addition of a patient information sheet to the consent form. Within the last year, there has been a further change, namely, the addition of a complaints clause,17 a common feature in consent forms for all research projects. The changes to the consent form have come about through discussion between the users, especially members of the DTU and the Human Ethics Committees of both the University of New South Wales and the University of Sydney. It has been pleasing to see ethics committees of other institutions, especially teaching hospitals, choosing to adopt this form rather than insisting on their own versions of it.
As mentioned previously, human fetal tissue has not been used therapeutically in Australia since the mid 1980s, but use may begin again in the near future for two reasons.
Firstly, fetal tissue obtained early in gestation (eg, at 7 weeks’ gestation) is less immunogenic than older tissue, especially that in the second trimester.18 Very recently, it has been shown that tissue obtained at this age will develop into entire organs, such as a kidney,18 or tissues which are functional, such as insulin-producing cells which can normalise blood glucose levels of mice with diabetes.19 It is conceivable that these tissues, with their low immunogenicity, may be used therapeutically.
Secondly, fetal tissue, especially from the gonads, may be a source of stem cells that could be converted into more mature cells, such as nerve cells, which may be trialled therapeutically for the treatment of Parkinson's disease and other neurological disorders.
Human fetal tissue is a scarce resource that has been used by numerous researchers at a dozen separate institutions from 1994 to 2002. With an average of 265 samples distributed annually during this time, researchers have conducted experiments and published 74 manuscripts in peer reviewed journals. Our understanding of the pathogenesis of human diseases has been advanced and more treatment options have been examined as a result. It is timely to give greater recognition to the benefits for which human fetal tissue can be used.
1: The number of fetuses and the number of human fetal tissue samples obtained each year between 1 January 1994 and 31 December 2002
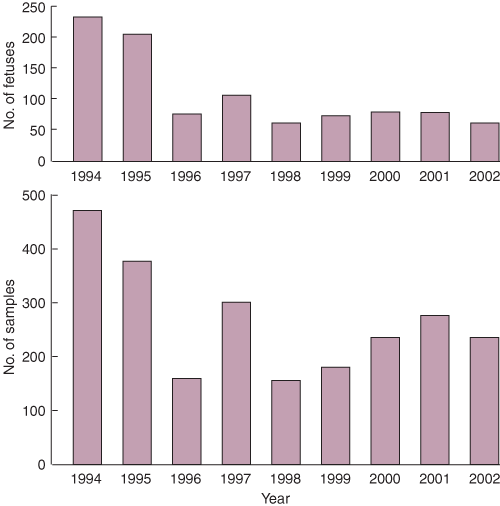
2: Use of human fetal tissue between 1 January 1984 and 31 December 2002
Tissue used |
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
2001 |
2002 |
||
Bone/cartilage (%) |
3 |
9 |
15 |
31 |
26 |
35 |
35 |
30 |
21 |
||
Eye (%) |
43 |
35 |
28 |
20 |
17 |
20 |
18 |
14 |
16 |
||
Kidney (%) |
5 |
11 |
19 |
25 |
19 |
24 |
13 |
9 |
11 |
||
Brain (%) |
27 |
18 |
9 |
5 |
9 |
8 |
6 |
11 |
14 |
||
Spinal cord (%) |
6 |
5 |
9 |
5 |
9 |
8 |
12 |
14 |
19 |
||
Pancreas (%) |
10 |
14 |
18 |
8 |
10 |
4 |
2 |
2 |
0.4 |
||
Liver (%) |
1 |
0.3 |
0 |
0 |
1 |
0 |
10 |
11 |
14 |
||
Other* (%) |
5 |
8 |
2 |
8 |
10 |
1 |
4 |
1 |
5 |
||
Total number of samples |
472 |
378 |
159 |
301 |
156 |
179 |
232 |
276 |
235 |
||
* Includes lung, spleen, lymph nodes, heart, adrenal glands, intestine, bladder, placenta, muscle and skin. |
|||||||||||
3: Research projects for which human fetal tissue has been used over the past decade*
Tissue type |
Research projects |
||||||||||
Bone/cartilage |
Development of bone and cartilage
|
||||||||||
Eye |
Vascularisation of the retina
|
||||||||||
Kidney |
Studies of mesangial cells, especially relating to diabetes |
||||||||||
Brain |
Cellular and developmental biology
|
||||||||||
Spinal cord |
Infection with viruses
|
||||||||||
Pancreas |
Dedifferentiation into pancreatic duct cells
|
||||||||||
Liver |
Interactions between pancreas and liver
|
||||||||||
Skin |
Feeder layers for growing human embryonic stem cells |
||||||||||
Placenta |
Biochemical studies |
||||||||||
Adrenal gland |
Biochemical studies |
||||||||||
Heart |
Developmental studies |
||||||||||
* This information was obtained from the researchers who receive human fetal tissue from the Diabetes Transplant Unit at the Prince of Wales Hospital, Sydney, and the University of Sydney. |
|||||||||||
- Bernard E Tuch1
- Hayley Scott2
- Muhammad T Tabiin3
- Liping P Wang4
- Patricia J Armati5
- 1 Diabetes Transplant Unit, Prince of Wales Hospital and University of New South Wales, Sydney, NSW.
- 2 Neurological Unit, School of Biological Sciences, University of Sydney, Sydney, NSW.
None identified.
- 1. Official Committe Hansard. Senate — legislation. Community Affairs Legislation Committee. Research involving embryos and prohibition of human cloning Bill 2002. September 17, 2002: 57-71. Available at: www.aph.gov.au/hansard/senate/commttee/s5776.pdf (accessed Oct 2003).
- 2. Maitland JE. Endocrine function of human foetal pancreas [MSc thesis]. Sydney: University of Sydney, 1980.
- 3. Tuch BE. Human fetal tissue for medical research. Med J Aust 1993; 158: 637-639.
- 4. Richards M, Fong C-Y, Chan W-K, et al. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nature Biotechnol 2002; 20: 933-936.
- 5. Smith D. Sydney team uses foetuses in stem cell study. Sydney Morning Herald 2002; 7 August: 1.
- 6. Tuch BE, Sheil ARG, Ng ABP, et al. Recovery of human fetal pancreas after one year of implantation in the diabetic patient. Transplantation 1988; 46: 865-870.
- 7. Guillemin GJ, Kerr SJ, Smythe GA, et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem 2001; 78: 1-13.
- 8. Slater M, Patava J, Kingham K, Mason RS. An immunoelectronmicroscopic study of the focal incorporation of growth factors into the extracellular matrix of osteoblast-like cells in vitro and the influence of 17 beta-estradiol. Am J Physiology 1994; 267: E990-E1001.
- 9. McLennan SV, Martell SY, Yue DK. Effects of meseangium glycation on matrix metalloproteinase activities: possible role in diabetic nephropathy. Diabetes 2002; 51: 2612-2618.
- 10. Penfold M, Armati PJ, Cunningham AL. Axonal transport of herpes simplex virions to epidermal cells. Evidence for a specialised mode of virus transport and assembly. Proc Nat Acad Sci U S A 1994; 91: 6529-6533.
- 11. Provis JM, Diaz-Araya CM, Dreher B. Ontogeny of the primate fovea: a central issue in retinal development. Prog Neurobiol 1998; 54: 549-580.
- 12. Chu Y, Hughes S, Chan-Ling T. Astrocyte precursor cell (APCs) and astrocyte differentiation in embryonic human retina: relevance to optic nerve colobomas. FASEB J 2001; 15: 2013-2015.
- 13. Si Z, Tuch BE, Walsh DA. Development of human fetal pancreas after transplantation into SCID mice. Cells Tissues Organs 2001; 168: 147-157.
- 14. Slobedman B, Mocarski ES. Quantitative analysis of latent human cytomegalovirus. J Virol 1999; 73: 4806-4812.
- 15. NHMRC Statement on human experimentation and supplementary notes, 1992. Supplementary note 5: The human fetus and the use of human fetal tissue. Canberra: NHMRC, 1992: 16-18.
- 16. Australian Health Ethics Committee. Ethical guidelines on the use of reproductive technology in clinical practice and research. Draft for public consultation February 2003. 18: Research on human fetuses and fetal tissues: 43-44. Available at: www.health.gov.au/nhmrc/issues/pdfcover/repro.htm (accessed Oct 2003).
- 17. Human ethics (University of Sydney). Sample 1: HREC [Human Research Ethics Committee] Approved Subject Information Statement and Consent Form for research involving the use of human fetal tissue in medical research. Patient information document. Use of human fetal tissue for medical research. Available at: www.usyd.edu.au/ethics/human/sample/dloads/Sample_1.rtf (accessed Oct 2003).
- 18. Dekel B, Burakova T, Arditti FD, et al. Human and porcine early kidney precursors as a new source for transplantation. Nature Med 2003; 9: 53-60.
- 19. Casting M, Peault B, Basmaciogullari A, et al. Blood glucose normalization upon transplantation of human embryonic pancreas into beta-cell-deficient SCID mice. Diabetologia 2001; 44: 2066-2076.





Abstract
Human fetal tissue is a scarce resource that has been used in Australia for biomedical research since 1980. From 1994 to 2002, it has been used for research by 19 biomedical researchers at 12 separate Australian institutions (four universities, six major teaching hospitals and two research institutes).
With an average of 265 samples distributed annually, researchers have conducted experiments in biomedical research with the approval of their Human Ethics Committees, and published 74 manuscripts in peer reviewed journals over the past decade.
The tissue is obtained from therapeutic termination of pregnancies at 8–20 weeks’, but mostly 14–18 weeks’, gestation. The average number of fetuses obtained over the past 10 years was 108 per annum.
Our understanding of the pathogenesis of human diseases such as diabetes, multiple sclerosis, retinopathy of prematurity and osteoporosis has been advanced because of such experiments, and better drug treatment of disorders such as osteoarthritis has been made possible with the use of human fetal tissue.
The benefits of human fetal tissue research need greater recognition.