Despite recent advances in antipsychotic agents, there remains a significant proportion of patients who do not respond well to pharmacological intervention. Such patients are commonly labelled "treatment resistant", despite little consensus as to the definition of the term.1
The label "treatment resistance" is used particularly to refer to patients whose positive symptoms of schizophrenia (including delusions and hallucinations) have not responded to treatment.1-3 (For definitions of "positive" and "negative" symptoms, see Lambert and Castle [page S57].4) The focus on positive symptoms has arisen largely because other domains were either not clinically well recognised or understood (eg, cognitive symptoms),5 or were considered to be unresponsive to treatment (eg, negative symptoms such as amotivation, apathy, social withdrawal, blunted affect and poverty of speech).6 Thus, pharmacological treatment for psychosis has been predominantly evaluated for its effect on positive symptoms,7 a narrow focus that may ignore other important outcomes such as community integration, quality of life or return to meaningful occupation.8 These latter outcome measures are particularly important for systems of care in Australia, with their emphasis on community-based treatment.
The prevalence of treatment resistance is hard to determine given the lack of agreement on defining the term. It has been estimated that 20%–45% of people with schizophrenia of over two years' duration are only partially responsive to antipsychotic medication,2,9 and 5%–10% of patients derive no benefit at all.1 However, these figures reflect treatment outcomes with first-generation ("typical") antipsychotics (FGAs). With second-generation ("atypical") antipsychotics (SGAs) now available, we need to reconsider what constitutes "non-response" (SGAs are further considered by Lambert and Castle [page S57]4).
There are a number of reasons for replacing the term "treatment resistance" with an alternative term. The use of "resistance" suggests that nothing can be done to improve schizophrenic symptoms and embeds a notion that the patient is resisting treatment rather than the illness itself being resistant to treatment. The "treatment resistance" label is no longer in tune with current therapeutic alternatives or with our more recent understanding of the basis of schizophrenia. Therefore, treatment resistance is better viewed as "incomplete recovery" (IR), a term reflecting the potential for improved therapeutic outcomes with the use of SGAs, which have been shown to be more effective than FGAs in treating certain domains of symptoms (Box 1).
Recent data from the Australian National Mental Health Survey of Psychotic Disorders suggest that the disabilities suffered by people with schizophrenia encompass all the symptom domains listed in Box 1.18 This supports emerging evidence that disability is less dependent on ongoing positive symptoms than is often assumed,8,19 and that interventions for IR need to focus on multiple symptom domains.
In the past, the main measure of treatment success has been the ability of FGAs to reduce positive symptoms. SGAs may confer an advantage to incompletely recovered patients by targeting a broader range of phenomena and associated disabilities that are important and perhaps critical to recovery.
Factors other than choice of medication that may influence a patient's recovery include individual factors relating to the patient, the illness and the treatment (Box 2).1 Particularly relevant to the management of the incompletely recovered patient are poor compliance with medication, concurrent illicit substance use (especially cannabis and psychostimulants), psychosocial stressors (eg, the home environment), physical comorbidity (which may be secondary to treatment — see Lambert et al [page S67]20), associated organic abnormality,21 prominent negative symptoms and neuropsychological deficits.5
Consideration of these factors may help the clinician to identify barriers to recovery. Ultimately, the relationship established between the doctor and the patient — the therapeutic alliance — will facilitate the process of recovery by helping to establish important details of the history, enhance compliance, engage the family, reduce stress, manage physical and psychological comorbidity, identify the role and degree of illicit substance misuse, and instil a sense of hope in the patient.
We discuss here some of the current thinking on medication strategies for managing incompletely recovered patients. Psychosocial strategies, which are also an integral part of managing these patients,22 are discussed in detail by Crosse (page 76).23
SGAs have the potential to reduce many psychopathological symptoms and are better tolerated than FGAs by most patients.17 However, for patients who respond poorly to both FGAs and SGAs, the evidence suggests that some SGAs may be more effective than others. Research on the efficacy of SGAs has focused mainly on their effect on positive symptoms, without fully examining the multidimensional nature of IR. Some SGAs may have particular relevance to certain symptom domains (Box 1), although the evidence base for these findings is often not derived from patients deemed to have IR, and few studies have directly compared the various agents. The limited comparative evidence available presents a complex picture that varies according to how symptoms and treatment goals are defined. For example, studies using strict definitions of resistant positive symptoms24-26 have found that the most appropriate medication for patients with non-responsive positive symptoms is clozapine;10,24,26 studies applying less stringent criteria suggest that other SGAs (eg, risperidone,27 olanzapine28,29) may also be effective for treating positive symptoms; olanzapine and risperidone may be more effective than clozapine for treating cognitive symptoms;12 and clozapine may be superior to olanzapine for reducing suicide attempts in patients at high risk.15 More detailed investigations of drug efficacy in each of the symptom domains are required.
A further consideration is that the time course for change in a particular domain will vary. For example, positive symptoms may improve within 4–6 weeks, while the domains of negative symptoms, cognition and social functioning may require 6–12 months for full benefit from treatment.
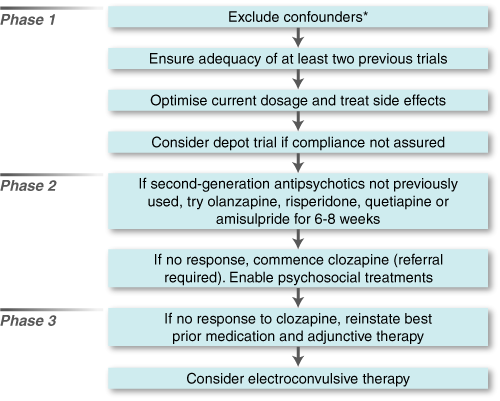
Pharmacological management of incompletely recovered patients can be considered in three phases (Box 3).
Phase 1: The clinician must first ensure that the patient truly meets criteria for IR. The patient's history should be reviewed to assess whether the patient is reluctant to accept or comply with treatment, or whether the medication itself is ineffective. With respect to the former, a number of confounders need to be explored (Box 2). Once these have been adequately dealt with, if IR is still considered likely a number of treatment history variables need to be considered.
Most guidelines for treating schizophrenia suggest that patients should have undergone a minimum of two trials in which they received 300–600 mg equivalents/day of chlorpromazine30 for 4–6 weeks31 with adequate adherence. Extra criteria concerning duration2 and intensity of symptoms24 have also been used. The next step involves making sure that the current treatment is optimised. All side effects, especially extrapyramidal symptoms, need to be minimised, as they may mimic IR (see Lambert and Castle, (page 67)4). The clinician must further ensure that the drug dose is within the optimal range, taking into account the curvilinear dose–response relationship of many drugs (eg, haloperidol, risperidone). Comorbid depression, a common finding in schizophrenia, may also confound the clinical picture. The use of an intramuscular depot antipsychotic can be useful when compliance has been confirmed to be problem. To date, however, depot preparations have been FGA drugs; more recent developments include long-acting forms of SGAs, with risperidone being the first such preparation approved for use in Australia.
At this point, if the patient is still not responding, phase 2 can be entered into.
Phase 2: If the patient has previously received only FGAs, an SGA should be tried (risperidone, olanzapine, quetiapine, or amisulpride). For treating positive symptoms, the SGA should be used within the clinically accepted therapeutic range (ie, generally 4–6 mg/day for risperidone, 15–25 mg/day for olanzapine, 450–1000 mg/day for quetiapine, 400–1200 mg/day for amisulpride), with a suggested duration of 6–8 weeks.
If the patient exhibits predominantly negative symptoms (and confounders such as depression and extrapyramidal symptoms have been managed), a longer course of treatment may be needed before concluding that the therapy is ineffective. Subtle but important changes in negative symptoms may take months to become manifest. In general, SGAs should be used in lower doses to treat negative symptoms (eg, 3 mg/day risperidone or 10 mg/day olanzapine). The pharmacology of amisulpride suggests that it is particularly effective against negative symptoms at a dose of 100–300 mg/day. As negative symptoms and executive neuropsychological deficits (eg, poor planning and problem-solving skills) both involve the same neural substrate in the prefrontal cortex,32 these lower doses may also be effective in treating cognitive symptoms.
Although the importance of affective symptoms in schizophrenia is starting to be recognised, optimal dosage and duration of drug therapy for treating affective disturbance are not yet established. The outcome of drug therapy may be a subtle stabilisation of mood rather than a major change in affective symptoms. It may be that longer-term treatment is necessary for effectiveness, although definitive research is still lacking in this area.
Improvements in the domain of social and role functioning are a consequence of achieving the best risk–benefit ratio for the individual. Cognitive and negative symptom improvements, with low side effects, may be at least as important as reduction in positive symptoms when it comes to role functioning.33 In the domain of social and role functioning, informed history from families provides the best index of improvement, which may be subtle and slow to become apparent. (For example, in patients receiving clozapine therapy, it is not uncommon to see improvements in this domain occurring 6–12 months, or longer, after commencement of therapy.) In circumstances in which comorbidity is significant, adjunctive therapy such as antidepressants may also play a role.
If there is no response to the other SGAs, clozapine is instigated. Clozapine remains unique in its ability to improve outcomes in patients with treatment-resistant positive symptoms.34 However, the drug's benefits must be weighed against its serious potential risks: neutropenia and agranulocytosis,35 weight gain and diabetes,36,37 epileptic seizures and cardiac problems such as cardiomyopathy.38 As clozapine may take some time to improve symptoms in domains such as social functioning, clinicians should consider a minimum trial period of six months. Commencement on clozapine is best undertaken within a specialist psychiatric setting, while maintenance of stabilised patients can be readily continued by general practitioners.
Phase 3: The third phase involves those whose positive symptoms ultimately do not respond even to an adequate trial (at least six months) of clozapine. At this stage, adjunctive drug treatment such as mood stabilisers (lithium and valproate), benzodiazepines (eg, clonazepam), propranolol, antidepressants and polyantipsychotic prescribing may be tried.1 It should be clearly noted that adjunctive strategies are not replete with evidence. They should be considered on an individual basis, with goals of treatment carefully defined and subsequently monitored so that inefficient polypharmacy is avoided. Such strategies usually require referral to a practitioner with special expertise in this area of pharmacotherapy. The use of electroconvulsive therapy (ECT) should also be considered for patients who have failed to respond to all other treatments.39 ECT may stabilise some patients and allow reinstigation of lower doses of medication to which the patient has shown partial prior response, or prepare the way for a more complete response to clozapine. In rare cases, maintenance ECT may be considered.40
1: Phenomenological domains of schizophrenia: targets for pharmacological intervention
Symptom domain |
Clinical features |
Comments |
|||||||||
Positive |
Delusions, hallucinations, formal thought disorder. |
SGAs ≥ FGAs. Clozapine has superior efficacy in patients with IR.10 |
|||||||||
Negative |
Avolition, apathy, anhedonia, affective blunting, poverty of speech. |
SGAs > FGAs. Few studies specifically examine negative symptoms as primary outcome measure; exception are studies of amisulpride.11 |
|||||||||
Cognitive5 |
Deficits in memory, attention, executive function (planning, flexible thinking, problem solving), judgement and insight. |
SGAs > FGAs. Different SGAs may have different effects on cognition.12-14 |
|||||||||
Affective |
Altered stability of mood (secondary dysthymia, depression, anxiety), manic-like symptoms. |
SGAs > FGAs. Some evidence that clozapine and olanzapine effective. |
|||||||||
Suicidality |
Suicidal ideation, suicidal behaviour. |
Clozapine > olanzapine.15 No evidence available for other SGAs and FGAs. |
|||||||||
Behavioural16 |
Social withdrawal (poor self-care, slowness, underactivity, lack of spontaneous speech); antisocial behaviour (hostility, aggression, unacceptable habits); incoherent and odd conversation. |
Limited research available to assess drug effects in this domain. |
|||||||||
Social and role functioning; quality of life |
Social interaction deficits; impaired activities of daily living (eg, self-care, paid employment, housing). |
More research required. Some suggestion that SGAs are superior to FGAs.17 |
|||||||||
FGA = first-generation antipsychotic. IR = incomplete recovery. SGA = second-generation antipsychotic. ">" = is more effective than. "≥" = are as effective as, or more effective than. |
|||||||||||
2: Confounding factors relevant in establishing and perpetuating incomplete recovery in patients with schizophrenia
Factor |
Relevant clinical issues |
||||||||||
Patient factors |
Illicit substance misuse |
||||||||||
|
Psychosocial milieu |
||||||||||
|
Physical comorbidity |
||||||||||
Illness factors |
Severity of psychopathology for each symptom domain (see Box 1) |
||||||||||
|
Poor prognosis of patients, who are typically single men with: |
||||||||||
|
Intellectual disability |
||||||||||
|
Marked cognitive impairment |
||||||||||
|
Poor premorbid adjustment |
||||||||||
|
Early and/or insidious onset of disorder |
||||||||||
|
Longer duration of prodrome |
||||||||||
|
Longer duration of untreated psychosis |
||||||||||
|
Negative symptoms at first admission |
||||||||||
|
Organic disorders (eg, temporal lobe abnormalities, brain injury) (suspected after abnormal CT scan, MRI scan or EEG) |
||||||||||
Treatment factors |
Non-compliance |
||||||||||
|
Side effects (eg, extrapyramidal symptoms, weight gain, diabetes) |
||||||||||
|
Incorrect dose |
||||||||||
|
Drug–drug interactions |
||||||||||
|
Delay in initiating treatment |
||||||||||
|
Drug bioavailability problems |
||||||||||
|
Inadequate rehabilitation program |
||||||||||
|
Poor therapeutic alliance between doctor and patient |
||||||||||
CT = computed tomography. EEG = electroencephalogram. MRI = magnetic resonance imaging. |
|||||||||||
- Christos Pantelis1
- Timothy J R Lambert2
- 1 Cognitive Neuropsychiatry Research and Academic Unit, Department of Psychiatry, University of Melbourne at Sunshine Hospital, St Albans, VIC.
- 2 OPEN (Office for Psychiatric Evaluation and Educational Newmedia), Department of Psychiatry, University of Melbourne, VIC.
CP has been on advisory boards for Bristol-Myers Squibb, Faulding, Sanofi and Novartis; has received funding for unrestricted research from Eli Lilly, Novartis, Janssen-Cilag, Bristol-Myers Squibb and AstraZeneca; and has received travel assistance to attend meetings from Eli Lilly, Novartis, Janssen-Cilag, AstraZeneca, Pfizer and Bristol-Myers Squibb. TJRL has been on advisory boards for Janssen-Cilag, Eli Lilly, Pfizer, Lundbeck, Sanofi, Novartis and Faulding; has received funding for unrestricted research from Eli Lilly, Novartis, Janssen-Cilag, Bristol-Myers Squibb, Pfizer and AstraZeneca; and has received travel assistance to attend meetings from Eli Lilly, Novartis, Janssen-Cilag and Bristol-Myers Squibb.
- 1. Pantelis C, Barnes TR. Drug strategies and treatment-resistant schizophrenia. Aust N Z J Psychiatry 1996; 30: 20-37.
- 2. Conley RR, Buchanan RW. Evaluation of treatment-resistant schizophrenia. Schizophr Bull 1997; 23: 663-674.
- 3. Kane JM. Treatment-resistant schizophrenic patients. J Clin Psychiatry 1996; 57(Suppl 9): 35-40.
- 4. Lambert TJR, Castle DJ. Pharmacological approaches to the management of schizophrenia. Med J Aust 2003; 178 Suppl May 5: S57-S61.<eMJA full text>
- 5. Pantelis C, Nelson HE, Barnes TRE. Schizophrenia: a neuropsychological perspective. London: John Wiley, 1996.
- 6. Liddle PF, Barnes TR. Syndromes of chronic schizophrenia. Br J Psychiatry 1990; 157: 558-561.
- 7. Lehman AF, Carpenter WT Jr, Goldman HH, Steinwachs DM. Treatment outcomes in schizophrenia: implications for practice, policy, and research. Schizophr Bull 1995; 21: 669-675.
- 8. Fossey EM, Harvey CA. A conceptual review of functioning: implications for the development of consumer outcome measures. Aust N Z J Psychiatry 2001; 35: 91-98.
- 9. Kane JM. Management strategies for the treatment of schizophrenia. J Clin Psychiatry 1999; 60(Suppl 12): 13-17.
- 10. Wahlbeck K, Cheine M, Essali A, Adams C. Evidence of clozapine's effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry 1999; 156: 990-999.
- 11. Speller JC, Barnes TR, Curson DA, et al. One-year, low-dose neuroleptic study of in-patients with chronic schizophrenia characterised by persistent negative symptoms. Amisulpride v. haloperidol. Br J Psychiatry 1997; 171: 564-568.
- 12. Bilder RM, Goldman RS, Volavka J, et al. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. Am J Psychiatry 2002; 159: 1018-1028.
- 13. Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull 1999; 25: 233-255.
- 14. Green MF. Interventions for neurocognitive deficits: editor's introduction. Schizophr Bull 1999; 25: 197-200.
- 15. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry 2003; 60: 82-91.
- 16. Harvey CR, Curson DA, Pantelis C, et al. Four behavioural syndromes of schizophrenia. Br J Psychiatry 1996; 168: 562-570.
- 17. Awad AG, Voruganti LN. Quality of life and new antipsychotics in schizophrenia. Are patients better off? Int J Soc Psychiatry 1999; 45: 268-275.
- 18. Jablensky A, McGrath J, Herrman H, et al. Psychotic disorders in urban areas: an overview of the Study on Low Prevalence Disorders. Aust N Z J Psychiatry 2000; 34: 221-236.
- 19. Gureje O, Herrman H, Harvey C, et al. The Australian National Survey of Psychotic Disorders: profile of psychosocial disability and its risk factors. Psychol Med 2002; 32: 639-647.
- 20. Lambert TJR, Velakoulis D, Pantelis C. Medical comorbidity in schizophrenia. Med J Aust 2003; 178 Suppl May 5: S67-S70.<eMJA full text>
- 21. Lubman DI, Velakoulis D, McGorry PD, et al. Incidental findings on brain magnetic resonance imaging in first-episode psychosis and chronic schizophrenia. Acta Psychiatr Scand 2002; 106: 331-336.
- 22. Castle DJ, Copolov DL, Wykes T. Pharmacological and psychosocial treatment of schizophrenia. London: Martin Dunitz, 2002.
- 23. Crosse C. A meaningful day: integrating psychosocial rehabilitation into community treatment of schizophrenia. Med J Aust 2003; 178 Suppl May 5: S76-S78.<eMJA full text>
- 24. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45: 789-796.
- 25. Conley RR, Tamminga CA, Bartko JJ, et al. Olanzapine compared with chlorpromazine in treatment-resistant schizophrenia. Am J Psychiatry 1998; 155: 914-920.
- 26. Conley RR, Tamminga CA, Kelly DL, Richardson CM. Treatment-resistant schizophrenic patients respond to clozapine after olanzapine non-response. Biol Psychiatry 1999; 46: 73-77.
- 27. Bondolfi G, Dufour H, Patris M, et al. Risperidone versus clozapine in treatment-resistant chronic schizophrenia: a randomized double-blind study. The Risperidone Study Group. Am J Psychiatry 1998; 155: 499-504.
- 28. Breier A, Hamilton SH. Comparative efficacy of olanzapine and haloperidol for patients with treatment-resistant schizophrenia. Biol Psychiatry 1999; 45: 403-411.
- 29. Tollefson GD, Birkett MA, Kiesler GM, Wood AJ. Double-blind comparison of olanzapine versus clozapine in schizophrenic patients clinically eligible for treatment with clozapine. Biol Psychiatry 2001; 49: 52-63.
- 30. Barnes TR, McEvedy CJ, Nelson HE. Management of treatment resistant schizophrenia unresponsive to clozapine. Br J Psychiatry Suppl 1996; Dec(31): 31-40.
- 31. Kane JM, Marder SR. Psychopharmacologic treatment of schizophrenia. Schizophr Bull 1993; 19: 287-302.
- 32. Pantelis C, Stuart GW, Nelson HE, et al. Spatial working memory deficits in schizophrenia: relationship with tardive dyskinesia and negative symptoms. Am J Psychiatry 2001; 158: 1276-1285.
- 33. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the "right stuff"? Schizophr Bull 2000; 26: 119-136.
- 34. Peuskens J. The evolving definition of treatment resistance. J Clin Psychiatry 1999; 60(Suppl 12): 4-8.
- 35. Copolov DL, Bell WR, Benson WJ, et al. Clozapine treatment in Australia: a review of haematological monitoring. Med J Aust 1998; 168: 495-497.
- 36. Newcomer JW, Haupt DW, Fucetola R, et al. Abnormalities in glucose regulation during antipsychotic treatment of schizophrenia. Arch Gen Psychiatry 2002; 59: 337-345.
- 37. Sussman N. Review of atypical antipsychotics and weight gain. J Clin Psychiatry 2001; 62(Suppl 23): 5-12.
- 38. Killian JG, Kerr K, Lawrence C, Celermajer DS. Myocarditis and cardiomyopathy associated with clozapine. Lancet 1999; 354: 1841-1845.
- 39. Chanpattana W, Chakrabhand ML, Kongsakon R, et al. Short-term effect of combined ECT and neuroleptic therapy in treatment-resistant schizophrenia. J ECT 1999; 15: 129-139.
- 40. Stevens J, Cheung P, Lambert T. Maintenance electroconvulsive therapy in schizophrenia. Aust N Z J Psychiatry 2001; 35: 132-133.





Abstract
Patients who fail to respond adequately to pharmacological treatment present an ongoing therapeutic challenge. The term "incomplete recovery" (IR) is preferred to the current term "treatment resistance" to describe these patients.
IR should be considered from a multidimensional perspective that includes a broad range of symptoms and functional disabilities that are relevant to schizophrenia.
The approach to the incompletely recovered patient needs to be systematic, with consideration given to the factors that may hamper recovery.
"Atypical" (second-generation) antipsychotic drugs target various domains of symptoms relevant to IR.
Adjunctive treatment strategies (eg, mood stabilisers, antidepressants, combinations of antipsychotics) may be useful, but should be undertaken in specialist psychiatric settings.
Although pharmacological treatment is a necessary first step in managing incompletely recovered patients, adjunctive psychosocial interventions are needed to optimise treatment success.