Australian immunisation guidelines state that a booster dose of tetanus vaccine should be given to patients with a tetanus-prone injury if 5 or more years have elapsed since their previous dose.1 Combined adult diphtheria tetanus (ADT) vaccine is preferred over tetanus toxoid vaccine because of the low level of population immunity to diphtheria.1
The main adverse reaction to ADT vaccination is pain at the injection site, which occurs in up to two-thirds of people and may last several days.1-3 Intramuscular administration produces less pain and local reaction than subcutaneous administration and is equally immunogenic in children and adolescents,3,4 and is recommended by the World Health Organization5 and Australian guidelines.1
Many health workers warm ADT vaccine before administration in the belief that this reduces pain and side effects. Warming does not alter the efficacy of ADT, as its components are stable for 2 weeks at 45oC.1 Methods of warming include leaving the vaccine vial at room temperature, holding it in a closed hand or rubbing it between the palms for a short period before use. However, there is no evidence that these practices are effective.
We aimed to determine:
The study was undertaken in the Emergency Department (ED) of a regional hospital, Wangaratta District Base Hospital, Victoria. The double-blind randomised controlled trial was approved by the ethics committee of the Hospital.
Thirty vials of ADT (CSL, Australia; containing diphtheria toxoid 2 Lf and tetanus toxoid 6 Lf per 0.5 mL, adsorbed on to aluminium phosphate, and thiomersal 0.01% w/v) were stored at 2–8°C in a temperature-monitored refrigerator. Ten nurses were randomly chosen to prepare three vials each:
With no deliberate warming ("cold");
Rubbed for 1 minute between the nurse's hands ("rubbed"); and
Placed in a 37°C warming cupboard for 5 minutes ("warmed").
Each vaccine was then prepared as if it were to be injected into a patient (vial checked with another nurse, vaccine drawn up with a blunt needle into a 2 mL syringe, and a 23-gauge hypodermic needle attached to the syringe) before injection into a 2 mL plastic microcentrifuge tube housed in a rack. The tube contained a wire flux temperature probe connected to a calibrated digital thermometer that provided instantaneous temperature measurements (TEK DTM 510, Tektronix Inc, Beaverton, Ore, USA). The experiment was conducted in the ED, which is air conditioned. Ambient temperature was also measured.
Temperature data were analysed by two-way analysis of variance.
Participants: A convenience sample of 150 patients, aged 16 years or older and requiring ADT booster vaccination according to Australian immunisation guidelines1 (Box 1), were recruited from the ED between April and December 2001. Participants were enrolled by ED nurses who considered each patient for whom ADT vaccine was ordered. Those who required inpatient treatment were excluded. Informed consent was obtained.
Intervention: Patients were randomly assigned to receive cold, rubbed or warmed ADT vaccine, using random numbers generated by computer before the study began. The method of vaccine preparation was concealed in an envelope, which was opened when the patient was ready to receive the vaccine. The vaccine was removed from storage at a temperature of 2–8°C and prepared by the nurse who was to administer it. Preparation was done in an area isolated from the patient, who was blinded to the method of preparation.
The vaccine was administered by registered nurses in the ED using a standardised technique (injection into the deltoid muscle at an angle of 60 degrees towards the shoulder with a 25 mm 23-gauge needle).1
Outcome: The primary outcome measure was the incidence of pain, with secondary outcomes being amount of pain and other adverse reactions. Outcomes were assessed at 5 minutes after injection and then by telephone after 24 and 48 hours, by nurses blinded to the method of vaccine preparation. Pain was scored using the McGill Present Pain Intensity Questionnaire (scale: 0 = no pain to 5 = excruciating pain).6 This scoring has been validated in other studies of pain after vaccination.7 Patients were reminded at follow-up to score only pain related to the injection and not other discomfort, and were also asked about other adverse reactions.
Statistical analysis: Data were analysed after study completion, using Minitab computer software.8 Incidence of pain was compared between the three vaccine-preparation groups using χ2 tests. For each patient who developed pain, pain scores were plotted over time, and "time-averaged pain" was determined by dividing the area under the curve by the follow-up time. This provided a representative pain score for each patient at any time during follow-up. These statistical techniques have been validated to assess serial measurements of pain.7,9 Time-averaged and peak pain scores were compared between groups by Kruskal–Wallis tests. Incidence of other adverse effects was qualitatively compared. Analyses of overall incidence of pain, and time-averaged and peak pain scores included only patients with complete follow-up.
With 80% power, an 0.05 level of significance and sample size of 50 for each arm, the randomised trial had the power to detect a difference in proportions of 0.23.
Vaccine temperature after preparation for simulated administration with different methods of warming is shown in Box 2. The vaccine that was not deliberately warmed ("cold") was significantly cooler than both the "rubbed" and "warmed" vaccines (P < 0.001 for each). The rubbed vaccine was also significantly, albeit slightly, cooler than the "warmed" vaccine (P < 0.001). Mean ambient temperature was 22°C (range, 95% CI, 21.6–22.4°C).
Numbers of patients at each stage of the trial are shown in Box 3. Patients in the three vaccine-preparation groups were similar in age, sex and weight (Box 4).
There was no significant difference in incidence of pain after ADT injection between the vaccine groups at any follow-up (Box 4). Overall incidence among those with complete follow-up was 56% (77/138). Among those with complete follow-up who had pain at some time, there was no significant difference in time-averaged or peak pain score (Box 4).
Other adverse reactions after ADT injection are also shown in Box 4. The numbers were too small for meaningful statistical analysis. Only one patient sought medical attention for adverse reactions. This patient received "cold" vaccine and developed a swollen injection site, which settled spontaneously over a week.
We found that warming ADT vaccine in a 37°C incubator or rubbing the vial between the palms for a minute did not reduce the incidence of pain after administration. This was possibly because, by the time it was ready to be administered, vaccine prepared straight from the refrigerator was only 8°C colder than "rubbed" vaccine and 10°C colder than vaccine pre-warmed to 37°C. Indeed, the temperature of all three vaccines approached ambient temperature, most likely because of the large surface area of the ADT vial and syringe compared with the small volume of vaccine (0.5 mL).
Rubbing the vaccine vial may have effects besides warming the vaccine, such as increasing the resuspension of vaccine components. However, the incidence of pain after rubbed vaccine was no different to that of cold or warmed vaccine.
Overall incidence of pain in our patients was 56%, which is consistent with previous reports.1-3 Other reported adverse effects after ADT vaccination were local and mostly mild, with no cases of serious immediate hypersensitivity reactions, which have an extremely low incidence.1,10,11
The double-blind nature of the study would have reduced reporting or measurement bias. We excluded patients who were lost to follow-up from the analysis to avoid assumptions about these patients' pain scores. This may have led to an underestimate of the incidence of pain, but it is unlikely to have affected the three groups differently.
A limitation of the study may be the way we quantified pain. The McGill Present Pain Intensity Questionnaire has been validated many times, including in assessment of pain after ADT vaccination,6,7 and was judged the most appropriate tool for assessing pain by telephone. Although assessment using a visual analogue scale may have been more sensitive, it was impractical in our circumstances.
Because of the high incidence of pain after ADT vaccination, our sample size of 50 was powerful enough to detect a relative change of 23% in pain incidence. However, recruiting more patients would have allowed more sensitive assessment of those few patients who develop more significant pain after vaccination (ie, pain score >2).
Although the study was conducted in an emergency department, we expect its results to be widely applicable in other settings, such as general practice and immunisation clinics. However, they are not necessarily applicable to other vaccines. For example, vaccines that are stored under refrigeration in pre-filled syringes would probably have less opportunity than ADT vaccine to warm up while being prepared for administration. Deliberate warming of these vaccines might be beneficial.
Our study illustrates that there is no difference in the incidence of pain after ADT vaccination regardless of whether ADT vaccine is prepared directly from the refrigerator, deliberately warmed to 37°C or rubbed between the palms.
1: Recommendations for ADT vaccination in wound management1
Past tetanus vaccination |
Time since last ADT |
Type of wound |
ADT required |
||||||||
3 doses |
< 5 years |
All wounds |
No |
||||||||
3 doses |
5–10 years |
Clean minor wounds |
No |
||||||||
3 doses |
5–10 years |
All other wounds |
Yes |
||||||||
3 doses |
> 10 years |
All wounds |
Yes |
||||||||
< 3 doses or uncertain |
|
All wounds |
Yes |
||||||||
ADT = adult diphtheria tetanus. |
|||||||||||
2: Temperature of adult diphtheria tetanus vaccine after different methods of warming
Method of warming |
Temperature (°C) (95% CI) |
||||||||||
No deliberate warming ("cold") |
19.1 (17.5–20.7) |
||||||||||
Rubbed for 1 minute ("rubbed") |
26.9 (24.5–29.3) |
||||||||||
Warmed to 37°C for 5 minutes ("warmed") |
28.9 (28.4–29.4) |
||||||||||
3: Patients at each stage of a trial of methods of warming ADT vaccine
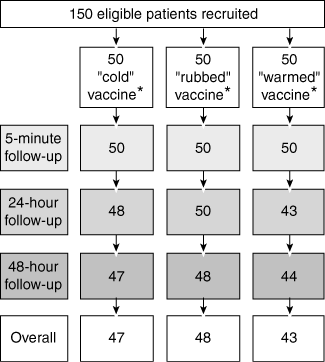
ADT = adult diphtheria tetanus. * All randomised patients received the intervention.
4: Characteristics of participants and outcomes of different methods of warming adult diphtheria tetanus vaccine
|
Cold (n = 50) |
Rubbed (n = 50) |
Warmed (n = 50) |
P |
|||||||
Participant characteristics |
|
|
|
|
|||||||
Median age (years) (range) |
40 (20–86) |
43 (18–87) |
43 (16–91) |
|
|||||||
Number of men |
33 |
30 |
29 |
|
|||||||
Median weight (kg) (range) |
75 (44–117) |
77 (40–121) |
77 (42–136) |
|
|||||||
Outcomes |
|
|
|
|
|||||||
Number with pain |
|
|
|
|
|||||||
5 minutes |
15/50 (30%) |
19/50 (38%) |
15/50 (30%) |
0.62 |
|||||||
24 hours |
15/48 (31%) |
15/50 (30%) |
17/43 (40%) |
0.58 |
|||||||
48 hours |
9/47 (19%) |
13/48 (27%) |
9/44 (20%) |
0.61 |
|||||||
Any time* |
26/47 (55%) |
27/48 (56%) |
24/43 (56%) |
0.99 |
|||||||
Median time-averaged pain score (IQR)*† |
0.50 (0.25–1.00) |
0.50 (0.25–1.00) |
0.50 (0.50–1.00) |
0.63 |
|||||||
Median peak pain score (IQR)*† |
1.0 (1.0–2.0) |
1.0 (1.0–2.0) |
1.0 (1.0–2.0) |
0.63 |
|||||||
Number with other adverse reactions |
|
|
|
|
|||||||
Swelling or lump |
6 |
2 |
8 |
NA |
|||||||
Red or warm |
4 |
1 |
3 |
NA |
|||||||
"Corked"‡ |
2 |
4 |
2 |
NA |
|||||||
Itch |
1 |
1 |
1 |
NA |
|||||||
IQR = interquartile range. NA = not analysed because of small numbers. * Among those who completed follow-up. † On a scale of 1–5 (1 = mild pain; 5 = excruciating pain). ‡ Term offered by patients, which seemed to represent the sensation of a muscular ache at the injection site. |
|||||||||||
Received 3 October 2002, accepted 11 March 2003
- Matthew J Maiden1
- Gregory N Benton2
- Russell A Bourne3
- Emergency Medicine, Wangaratta District Base Hospital, Wangaratta, VIC.
We thank the nursing staff at Wangaratta Hospital Emergency Department for collecting the patient data. Thanks to Geelong Hospital and Dr Julian Stella for their assistance with statistical analysis and manuscript preparation.
CSL Ltd donated vials of vaccine for the in-vitro study but had no other involvement with the study, analysis or publication of the results.
- 1. National Health and Medical Research Council. The Australian immunisation handbook. 7th ed. Canberra: NHMRC, 2000.
- 2. Update: vaccine side effects, adverse reactions, contraindications, and precautions. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1996; 45(RR-12): 1-35.
- 3. Mark A, Carlsson RM, Granstrom M. Subcutaneous versus intramuscular injection for booster DT vaccination of adolescents. Vaccine 1999; 17: 2067-2072.
- 4. Ipp MM, Gold R, Goldbach M, et al. Adverse reactions to diphtheria, tetanus, pertussis-polio vaccination at 18 months of age: effect of injection site and needle length. Pediatrics 1989; 83: 679-682.
- 5. World Health Organization. Immunisation in practice: a guide for health workers who give vaccines. Oxford: Oxford University Press, 1989; 3: 117-168.
- 6. Melzack R. The McGill pain questionnaire: major properties and scoring methods. Pain 1975; 1: 277-299.
- 7. Su L, Tucker R, Frey SE, et al. Measuring injection-site pain associated with vaccine administration in adults: a randomized, double-blind, placebo-controlled clinical trial. J Epidemiol Biostat 2000; 5: 359-366.
- 8. Minitab statistical software. Release 12. State College, Pa: Minitab Inc, 1998.
- 9. Matthews JNS, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990; 300: 230-235.
- 10. Jacobs RL, Lowe RS, Lanier BQ. Adverse reactions to tetanus toxoid. JAMA 1982; 247: 40-42.
- 11. Stratton KR, Howe CJ, Johnston RB, editors. Adverse events associated with childhood vaccines: evidence bearing on causality. Washington, DC: National Academy Press, 1994: 100-109.





Abstract
Objective: To determine whether warming or rubbing adult diphtheria tetanus (ADT) vaccine immediately before administration affects its temperature and reduces the incidence of pain.
Design: Double-blind, randomised controlled trial and in-vitro temperature study.
Setting: Emergency department (ED) of a regional hospital between April and December 2001.
Patients: Convenience sample of 150 patients aged 16 years or over who presented to the ED requiring ADT booster vaccination.
Intervention: Patients were randomised to receive vaccine that was "cold" (no deliberate warming), "rubbed" between the palms for 1 minute, or "warmed" in a 37°C incubator; vaccine was administered as recommended in Australian guidelines.
Main outcome measures: Incidence of pain and pain score on McGill Present Pain Intensity Questionnaire at 5 minutes, 24 hours and 48 hours after injection; and temperature of vaccine after preparation for simulated administration.
Results: The "cold" vaccine had significantly lower temperature (mean, 19.1°C; 95% CI, 17.5–20.7°C) than the "warmed" vaccine (mean, 28.9°C; 95% CI, 28.4–29.4oC) and "rubbed" vaccine (mean, 26.9°C; 95% CI, 24.5–29.3°C). There was no significant difference in incidence of pain between the groups who received vaccine prepared in different ways at any follow-up (5 min: P = 0.62; 24 h: P = 0.58; 48 h: P = 0.61) or overall (P = 0.99). Among those who completed follow-up, incidence of pain at any time was 77/138 (56%); there was no difference in their time-averaged pain scores (P = 0.63) or peak pain scores (P = 0.60).
Conclusions: Warming or rubbing ADT vaccine does not reduce the incidence of pain after administration. Regardless of how ADT vaccine is prepared, its temperature approaches ambient by the time it is injected.