In Australia, 90 000 Aboriginal and Torres Strait Islanders live in remote communities, many of which are accessible only by aircraft, especially in the wet season. Most of the people in these communities have much worse social and economic circumstances, education, living conditions and health status than other Australians.1-3
Chronic hepatitis B virus (HBV) infection is endemic in Aboriginal and Torres Strait Islander communities. Studies in the 1980s and early 1990s showed that 46.9% of Aboriginal schoolchildren in rural and urban areas of the Northern Territory (NT) have serological markers of HBV infection,4 and up to 26% of rural Aboriginal populations are positive for hepatitis B surface antigen (HBsAg).5-7 This suggests that most of the transmission of HBV infection occurs at an early age, including in schools.4 As hepatitis B vaccination was introduced in 1988 for NT Aboriginal children, and for all NT children in 1990, there will be a need for appropriate management of chronic HBV infection in remote NT Aboriginal communities for decades to come.
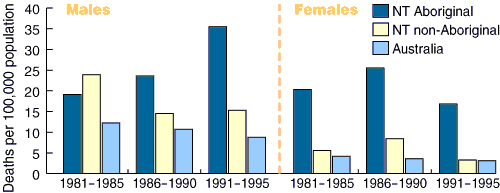
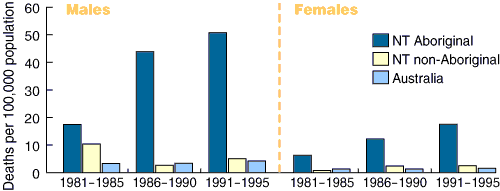
In 1991–1995, the death rates (for all causes of chronic liver disease and cirrhosis) were four times and 5.5 times higher for Aboriginal men and women, respectively, compared with rates for the general Australian population (Box 1).8 Furthermore, the age-adjusted death rates for liver cancer for NT Aboriginals have almost tripled in the period 1981–1995 (Box 2). However, the numbers are small (maximum of eight cases per year recorded in 1995), and may or may not reflect a true increase. Hepatocellular carcinoma (HCC) may occur on a background of chronic HBV infection. One study in patients with HCC, in whom serological tests were performed, showed HBsAg positivity in seven of eleven Aboriginal patients (63.6%) and two of four non-Aboriginal patients. Their median and mean age was 59 years.9 Thus, controlling HBV infection will have a big impact on HCC, even though the numbers are small. Australian Aboriginals are 12 times more likely to die of liver cancer than the general population.3 During the period 1987–1997, primary liver cancer was the third most common cancer and the second-highest cause of cancer death in Aboriginal men.3
Optimal management of chronic HBV infection is a constant source of concern for those providing healthcare services in remote areas. Yet, in this population, no guidelines exist to facilitate follow-up, appropriate and timely referral, and further investigation of HBV infection.10
Guidelines to assist primary healthcare providers in the broader community to deliver best-practice care to patients with HBV infection have been published.11-13 They assist with testing and indications for specialist referral, but do not cover screening for HCC, because of a lack of evidence for its value.
This update applies current opinion and available evidence regarding local follow-up, referral for treatment and screening for HCC to the context of remote-dwelling Aboriginals and Torres Strait Islanders, who have many different needs compared with other Australian subpopulations. Inherent in the care of patients with HBV infection is the need for staging and surveillance of asymptomatic patients.
Primary prevention, through education, counselling and vaccination, remains the mainstay of combating HBV infection and, for those infected, avoidance of exacerbating hepatic insults, particularly alcohol, is crucial. Recently, in Australia, there has been increasing use of more sophisticated therapy, including antiviral medication, for those with progressive hepatic inflammation and fibrosis, with the aim of preventing progression to decompensated liver disease or HCC, which may occur in 25% of patients.14 Other developments relate to the options available for management of HCC, especially when it is diagnosed early before symptoms manifest.15
Ninety per cent of individuals infected with HBV at birth or in early childhood will develop chronic HBV infection. Each year, in 1%–10% of those infected, seroconversion occurs (from HBeAg positive to HBeAg negative), with 10% remaining HBeAg positive throughout their life. The remainder (the majority) are HBsAg positive and HBeAg negative.12,14
Of those remaining HBsAg positive, around 25% will progress to end-stage liver disease. In patients with chronic infection, there is a 200-fold increased risk of HCC, causing death in 10%.16,17 The risk of a patient developing sequelae of HBV-associated cirrhosis is higher in men, those infected at a younger age and those with concurrent hepatitis C or HIV infection. Alcohol misuse is also associated with a greater risk of disease progression.
Survival in patients with HBV cirrhosis is 71% at 5 years, with death being due to bleeding, sepsis or HCC.18 In patients with untreated HCC, survival beyond 2 years is uncommon.
Patients with chronic HBV infection who are HBV-DNA negative and have normal alanine aminotransferase (ALT) levels have little liver-related mortality. A longitudinal study of 92 such patients over 15 years showed no liver-related deaths.19
Interferon-α was the first drug to become available to prevent progression of liver disease. Success, defined as loss of HBeAg, elimination of detectable virus from the blood (HBV-DNA negative) and normalisation of liver function tests, occurs in 40% of those treated with a 6-month course. Interferon-α is administered by subcutaneous injection and is associated with many adverse side effects. Since the introduction of lamivudine, the role of conventional interferon-α monotherapy in the treatment of HBV infection has decreased significantly.
Lamivudine, a nucleoside analogue used extensively in the management of HIV infection, and now available for patients with active chronic HBV infection, is taken as a daily tablet. It is well tolerated, with few side effects, infrequently reported. It has a cumulative benefit, with the seroconversion rate (from HBeAg positive to HBeAg negative) up to 73% at 4 years.20 Those most likely to respond have high ALT levels and low levels of viraemia, together with a liver biopsy result showing a high degree of activity (based on the degree of inflammation and necrosis and the stage of fibrosis). To receive treatment, patients need to have serial blood tests performed that show an elevated serum ALT level and HBV viraemia. They are required to consume little alcohol for 6 months, and then undergo a liver biopsy. Success is defined as elimination of virus from the blood and normalisation of ALT levels, at which time treatment may stop. Survival of patients treated successfully is significantly greater than in those not treated and those treated in whom HBeAg seroconversion does not occur.21
Studies have shown that patients with HBV infection undergoing immunosuppressive therapy for renal transplantation or chemotherapy have a decreased risk of hepatitis flare when taking lamivudine.22
There is concern over the selection of resistant strains.20 Extrapolating from experience with antiviral management of HIV, higher rates of development and selection of resistant mutations to lamivudine may occur in those with intermittent adherence to treatment.
Adefovir dipivoxyl has recently become available via compassionate access for patients with severe disease reactivation due to the lamivudine-resistant (YMDD) strain. Future treatment options likely to become available include other nucleoside analogues and more acceptable preparations of interferon (pegylated), plus combination approaches. Currently, all antivirals used to treat HBV infection require initiation and monitoring in a hospital-based liver clinic. Lamivudine and interferon are funded via the Pharmaceutical Benefits Scheme S100 category.
Transplantation for end-stage and decompensated HBV-associated cirrhosis is now widely accepted following the recognition of improved graft survival with administration of lamivudine and specific HBV immunoglobulin. Approval for transplantation involves extensive assessment of a variety of patient factors. Once accepted, the patient must live close to a major centre and be available to respond immediately to the availability of a donor organ. Waiting times are generally in the order of 3–9 months and dependent largely on blood-group status. After the operation, the patient is required to remain close to the hospital for some months. Regular specialist follow-up is life-long and immunosuppressive drugs must be taken daily. Significant adverse side effects include the risk of sepsis. Management is complicated, especially when there are comorbidities, such as renal disease, hypertension and diabetes.
Hepatologists often screen for HCC by regular measurement of serum α-fetoprotein levels and liver ultrasound examination. However, there is no widely published and accepted screening protocol. Early identification gives a greater opportunity for curative therapy, but no randomised controlled trial has shown benefits from surveillance. Screening has been recommended for those with clinical or biopsy-proven cirrhosis,23 and a 16-year longitudinal study of screening for HCC in Alaskan Indigenous people with HBsAg positivity showed a benefit from measuring α-fetoprotein levels only.24 While this is a reasonable compromise, the highest yield comes from measuring α-fetoprotein levels and performing ultrasound examinations.25
Early diagnosis of HCC allows for a variety of treatment options aimed at cure as well as prolonging life and improving quality of life. Treatment modalities range from transplantation to radio- and microwave-frequency ablation, resection and hemihepatectomy, intra-arterial chemotherapy and embolisation, and percutaneous intralesional ethanol injections, each requiring an extensive work-up and attendance at tertiary referral hospitals.15
In the context of remote-dwelling Aboriginals and Torres Strait Islanders, management of chronic HBV infection must take into account all individual and population healthcare issues. Any decision to screen, investigate and treat requires justification, as does a decision not to offer "Australian standard care". In principle, those infected should be offered the same standard of care as provided in urban centres. Regrettably, however, the poorer health status and reduced life expectancy of Australia's Indigenous population results from conditions with greater priority than HBV infection, such as diabetes, cardiovascular disease and renal failure. Furthermore, travel from remote areas for a "routine" liver ultrasound examination may not be acceptable to patients who have no relevant symptoms.
It is important for healthcare providers and communities to prioritise resources. Excessive attention to chronic HBV infection could divert resources away from programs which may have a more significant impact on the health of Indigenous people. There is also a risk that if complicated resource-intensive protocols are created, and are not adhered to by primary healthcare providers, then those who would most benefit from screening would not be appropriately monitored. Regular blood tests are already performed as part of check-ups for "well" men and women and chronic disease management. We believe that, in this context, screening should be by regular α-fetoprotein measurements, reserving further investigation, including ultrasound, for those who have abnormal results of blood tests, including abnormal liver enzyme levels.
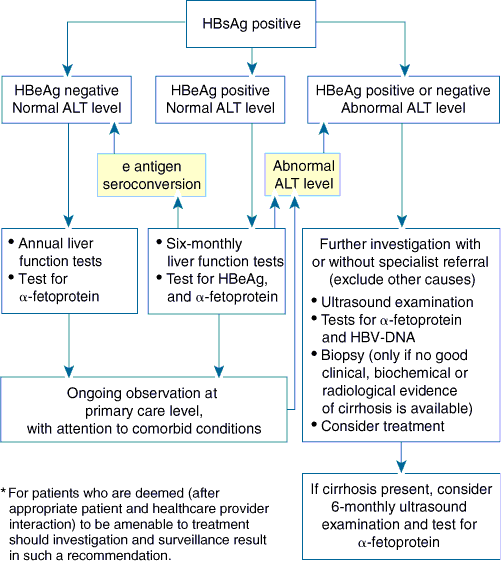
We recommend that patients be monitored according to the algorithm given in Box 3. Individual patients need to understand the purpose of follow-up, which is to decrease the risk of progression to decompensated liver disease and HCC. Patients being screened and monitored will require a good understanding of the reasons for investigation and consideration of treatment, particularly as they are likely to be asymptomatic at the appropriate time for intervention. Regular clinical review and modification of other lifestyle factors, particularly alcohol intake, should take priority over biochemical testing. Other reasons for abnormal liver function tests should also be kept in mind. These include diabetes, obesity, dyslipidaemia, medications, alcohol and kava consumption.
We recommend that primary healthcare providers adopt a flexible approach in undertaking follow-up of remote-dwelling Aboriginal and Torres Strait Islander patients with chronic HBV infection. For instance, the intensity of follow-up, or even whether a patient is followed up or not, might depend on a number of factors:
patient choice, if appropriate decision-making opportunities are ensured;
patient willingness to adhere to follow-up;
lifestyle issues, including alcohol consumption;
existence of significant comorbidities (including age) altering a patient's prognosis; and
a belief after discussion with the patient, and other people of significance to the patient, that treatment options would be culturally or socially inappropriate, even if screening showed significant early asymptomatic liver disease.
- Dale A Fisher1
- Sarah E Huffam2
- Royal Darwin Hospital, Casuarina, NT.
We thank Professor Geoff McCaughan, Dr Alex Brown, Dr Tarun Weeramanthri, Dr Steven Skov, Dr John Condon, Associate Professor Ian Anderson and the reviewers for the Central Australia Rural Practitioners Association (CARPA) guidelines for their valuable review and contributions to this article.
None identified.
- 1. Condon JR, Warman G, Arnold L, editors. The health and welfare of Territorians. Darwin: Epidemiology Branch, Territory Health Services, 2001. Available at: http://www.nt.gov.au/health/health_gains/epidemiology/welfare_territorians.pdf (accessed Nov 2002).
- 2. Australian Bureau of Statistics and Australian Institute of Health and Welfare. The health and welfare of Australia's Aboriginal and Torres Strait Islander peoples. Canberra: ABS, 2001. (Catalogue No. 4704.0.) Available at: http://www.abs.gov.au/Ausstats/abs@.nsf/Lookup/8D02FB93FD87ECDACA256D910000AD80 (link updated Nov 2005).
- 3. Cunningham J, Paradies Y. Mortality of Aboriginal and Torres Strait Islander Australians 1997. 3315.0. 17-4-2000. Canberra, Australian Bureau of Statistics. Occasional Paper. Available at: http://www.abs.gov.au/ausstats/abs%40.nsf/9cfdfe271b7930bbca2568b5007b8618/7dfa0774613c8e16ca2568ba001960ca!OpenDocument (accessed Nov 2002).
- 4. Gardner ID, Wan X, Simms PA, et al. Hepatitis B virus markers in children and staff in Northern Territory schools. Med J Aust 1992; 156: 638-641.
- 5. Burrell CJ, Cameron AS, Hart G, et al. Hepatitis B reservoirs and attack rates in an Australian community. A basis for vaccination and cross-infection policies. Med J Aust 1983; 2: 492-496.
- 6. Holman CDJ, Bucens MR, Quadros CF, Reid PM. Occurrence and distribution of hepatitis B infection in the Aboriginal population of Western Australia. Aust N Z J Med 1987; 17: 518-525.
- 7. Campbell DH, Sargent JW, Plant AJ. The prevalence of markers of infection with hepatitis B virus in a mixed race Australian community. Med J Aust 1989, 150: 489-492.
- 8. Dempsey KE, Condon JR. Mortality in the Northern Territory 1979-1997, Darwin: Territory Health Services, 1999. Available at: http://www.nt.gov.au/health/health_gains/epidemiology/mortality_NT_1979_1997.pdf (accessed Nov 2002).
- 9. Wan X, Mathews JD. Primary hepatocellular carcinoma in Aboriginal Australians. Aust J Public Health 1994; 18: 286-290.
- 10. Scrimgeour D. Screening Issues for Aboriginal people. CARPA Newsletter No. 24. Oct 1996, 4-10.
- 11. Farrell GC. Chronic viral hepatitis [Practice essentials – gastroenterology]. Med J Aust 1998; 168: 619-626.
- 12. Gastroenterological Society of Australia. Hepatitis B. New tests, new treatments, new recommendations. An information booklet for health care providers from the Digestive Health Foundation. 2nd edition. Sydney: Gastroenterological Society of Australia, 2000.
- 13. Asian Pacific Guidelines on the prevention and management of hepatitis B (core working party for Asia-Pacific Consensus on hepatitis B and C). Consensus statements on the prevention and management of hepatitis B and C in the Asia-Pacific region. J Gastroenterol Hepatol 2000; 15: 825-841.
- 14. Mahoney FJ. Update on diagnosis, management and prevention of hepatitis B virus infection. Clin Microbiol Rev 1999: 12: 351-366.
- 15. Badvie S. Hepatocellular carcinoma. Postgrad Med J 2000; 76: 4-11.
- 16. Beasley PR, Hwang LY, Lin CC. Hepatocellular carcinoma and hepatitis B virus: a prospective study of 22707 men in Taiwan. Lancet 1981; 2: 1129-1133.
- 17. Farrell GC. Towards the eradication of hepatitis B in Australia. Aust N Z J Med 1988; 18: 645.
- 18. De Jongh FE, Janssen HLA, de Man RA. Survival and prognostic indicators in hepatitis B surface antigen positive cirrhosis of the liver. Gastroenterology 1992; 103: 1630-1635.
- 19. de Franchis R, Meucci G, Vecchi M, et al. The natural history of asymptomatic hepatitis B surface antigen carriers. Ann Intern Med 1993; 118: 191-194.
- 20. Strasser SI, McCaughan GW. Therapies for chronic hepatitis B: emerging roles for nucleoside analogues. Aust N Z J Med 2000; 30: 556-558.
- 21. Niederau C, Heintges T, Lange S, et al. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N Engl J Med 1996; 334: 1422-1427.
- 22. Han DJ, Tae HK, Park SK, et al. Results on preemptive or prophylactic treatment of lamivudine in HBsAg (+) renal allograft recipients: comparison with salvage treatment after hepatic dysfunction with HBV recurrence. Transplantation 2001; 71: 367-394.
- 23. Llovet JM, Bruix J. Early diagnosis and treatment of hepatocellular carcinoma. Baillieres Best Pract Res Clin Gastroenterol 2000; 14: 991-1008.
- 24. McMahon BJ, Bulkow L, Harpster A, et al. Screening for hepatocellular carcinoma in Alaskan natives infected with chronic hepatitis B: a 16-year population based study. Hepatology 2000; 32: 842-846.
- 25. Lin D, Liaw Y. Optimal surveillance of hepatocellular carcinoma in patients with chronic viral hepatitis. J Gastroenterol Hepatol 2001; 16: 553-559.





Abstract
Chronic HBV infection is common in remote Aboriginal and Torres Strait Islander communities, where resources are scarce and patients may have several concurrent illnesses.
The management of chronic HBV infection has changed over recent years, with greater application of serological and radiological investigations and new, more acceptable treatments for chronic liver disease, cirrhosis and hepatocellular carcinoma.
Optimal follow-up procedures for patients with chronic HBV infection are still being debated, but may not be applicable to Aboriginal and Torres Strait Islander communities where factors such as endemicity, remoteness, frequent comorbidities, shorter life expectancy and cultural differences in health priorities must be taken into consideration.
We have defined an algorithm to assist primary care providers caring for patients with chronic HBV infection in Aboriginal and Torres Strait Islander communities. Patients are divided into one of three categories for follow-up and referral based on clinical features, and results of liver enzyme and serological tests.