The major role of antiplatelet drugs in clinical practice is to prevent the adverse clinical sequelae of thrombosis in atherosclerotic arteries to the heart (acute coronary syndromes [ACS]), brain (ischaemic stroke), and limbs (intermittent claudication and rest pain); and thrombosis of stagnant blood in veins (venous thromboembolism) and heart chambers (atrial fibrillation, heart failure).
The pivotal role of platelets in thrombosis is illustrated in Box 1.1
Aspirin, clopidogrel, dipyridamole and the glycoprotein IIb/IIIa receptor antagonists (abciximab and tirofiban) are antiplatelet drugs approved for use in Australia (see Box 2).
Mechanism of action: Aspirin (acetylsalicylic acid) irreversibly inhibits prostaglandin H synthase (cyclooxygenase-1) in platelets and megakaryocytes, and thereby blocks the formation of thromboxane A2 (TXA2; a potent vasoconstrictor and platelet aggregant).3 It is only the parent form, acetylsalicylic acid, which has any significant effect on platelet function. Because platelets are unable to regenerate cyclooxygenase, the immediate antithrombotic effect of aspirin remains for the lifespan of the platelet (8–10 days). As, after stopping aspirin therapy, normal haemostasis may be regained when about 20% of platelets have normal cyclooxygenase activity, daily aspirin intake is recommended.
Dose and administration: Formulations of aspirin currently available in Australia include a 100 mg enteric-coated form as well as 300 mg and 324 mg soluble tablets.
Aspirin is rapidly absorbed from the gastrointestinal (GI) tract, with peak concentrations achieved in 30–40 minutes.
When given as a single oral dose, at least 160 mg of soluble aspirin is required to maximally inhibit platelet function within 30 minutes. Thus, this dose (half 324 mg tablet) should be given as a loading dose if a rapid antiplatelet effect is required.
Soluble aspirin (40–80 mg daily) and enteric-coated aspirin (80–100 mg daily) have a cumulative effect, so that platelet TXA2 formation is maximally inhibited (by more than 95%) after 4–5 days.3,4
The evidence from randomised controlled trials (RCTs) supports daily doses of aspirin in the range 75–150 mg for the long-term prevention of serious vascular events in high risk patients (E1; Box 3).5 Higher doses of 500–1500 mg aspirin daily are no more effective (E1)5 (for an explanation of level-of-evidence codes, see Box 4).
Adverse effects: Aspirin use is associated with dose-related symptoms of upper-GI toxicity (nausea, heartburn, epigastric pain). High doses of aspirin (500–1500 mg daily) compared with medium (75–325 mg daily) or low (30 mg) doses, significantly increase the risk of upper-GI symptoms (E1).12-15 Enteric-coated aspirin may cause less gastric irritation than soluble aspirin.4
Aspirin is associated with about a 60%–70% excess of non-fatal extracranial haemorrhage (mostly from the GI tract), which corresponds to an absolute excess risk of about one or two per 1000 patients treated per year (E1).5,16 The risk of bleeding is not significantly different with different daily aspirin doses or different aspirin formulations (plain, enteric-coated and buffered aspirin).12,16 Aspirin is associated with an increased risk of intracranial haemorrhage of about one per 1000 patients treated for 3 years (E1).17 Again, there is no clear variation in risk with the dose of aspirin used (E1).17
Mechanism of action: The thienopyridine derivatives (clopidogrel and ticlopidine) are metabolised in the liver to active compounds which covalently bind to the adenosine phosphate (ADP) receptor on platelets and dramatically reduce platelet activation (see Box 1).
Dose and administration: An oral loading dose of 300–600 mg clopidogrel produces detectable inhibition of ADP-induced platelet aggregation after 2 hours, which becomes maximal after 6 hours.18,19 If a loading dose of clopidogrel is not used, repeated daily oral doses of 75 mg clopidogrel are required to achieve a steady-state maximal platelet inhibition, which is comparable with that produced by 250 mg ticlopidine orally, twice daily.20
Adverse effects: Compared with aspirin, the thienopyridines are associated with a lower risk of GI haemorrhage (odds ratio [OR], 0.71; 95% CI, 0.6–0.9) and upper-GI symptoms (OR, 0.84; 95% CI, 0.8–0.9), and an increased risk of diarrhoea and of skin rash (E1).21 Ticlopidine doubles the risk of skin rash (OR, 2.23; 95% CI, 1.7–2.9) and diarrhoea (OR, 2.27; 95% CI, 1.9–2.8) compared with aspirin (E1), whereas clopidogrel increases skin rash (OR, 1.32; 95% CI, 1.2–1.5) and diarrhoea by about a third (OR, 1.34; 95% CI, 1.2–1.6), compared with aspirin (E2).21 Clopidogrel has superseded ticlopidine because the latter is associated with an excess of neutropenia compared with aspirin (OR, 2.72; 95% CI, 1.5–4.8) (E1), particularly in the early months of therapy, whereas clopidogrel is not (OR, 0.63; 95% CI, 0.3–1.4) (E2).21 Furthermore, ticlopidine is associated with a significant excess of thrombocytopenia and of thrombotic thrombocytopenic purpura (TTP) (E32),22,23 whereas clopidogrel is not (E2).6,21 Although TTP has been reported in 20 patients taking clopidogrel,24,25 the association between clopidogrel and TTP is likely to be coincidental (E32).26 Therefore, if ticlopidine is to be used, haematological monitoring should be undertaken at commencement and every 2 weeks in the first 4 months of therapy.
Dipyridamole inhibits phosphodiesterase, which inactivates cyclic AMP (Box 1). Increased intraplatelet concentrations of cyclic AMP reduce the activation of cytoplasmic second messengers. Dipyridamole also stimulates prostacyclin release and inhibits thromboxane A2 formation. Because the effect is short-lasting, repeated dosing or slow-release preparations are required to inhibit platelet function for 24 hours.
Mechanism of action: Glycoprotein IIb/IIIa receptor antagonists block the final common pathway for platelet aggregation (Box 1). Abciximab is a humanised mouse antibody fragment with a high binding affinity for the glycoprotein IIb/IIIa receptor. Tirofiban (a non-peptide derivative of tyrosine) and eptifibatide (a synthetic heptapeptide) mimic part of the structure of fibrinogen that interacts with the glycoprotein IIb/IIIa receptor and thus compete with ligand binding of fibrinogen to the glycoprotein IIb/IIIa receptor. Eptifibatide is not currently approved for use in Australia.
Dose and administration: Glycoprotein IIb/IIIa receptor antagonists are given intravenously as a bolus injection, followed by a continuous infusion for up to 72 hours. At 24 hours after cessation of an infusion of abciximab, there is persistent blockade of more than 50% of platelet glycoprotein IIb/IIIa receptors, but platelet function recovers after 2 days. By contrast, the antiplatelet effects of tirofiban rapidly dissipate after cessation of the infusion.
Adverse effects: Thrombocytopenia is relatively common (1.6% compared with 0.7% for placebo), and can be delayed for up to 5 days (E2). Acute severe thrombocytopenia (< 20 x 109/L in 24 hours) occurs in 0.6% of patients, and needs to be differentiated from other causes such as immune heparin-induced thrombocytopenia (E2). The risk of recurrent thrombocytopenia is increased with re-exposure to abciximab.27
A summary of indications for antiplatelet drugs and appropriate regimens is given in Box 5, and new messages about antiplatelet therapy are summarised in Box 6. Messages for patients are outlined in Box 7.
In the absence of contraindications, immediate treatment with 160 mg aspirin is appropriate for all patients with suspected acute ischaemic syndromes of the brain, heart and limbs, including those undergoing percutaneous interventions (E1).5 Long-term treatment (with 75–150 mg aspirin daily) is indicated thereafter (E1).5 Treating 1000 patients at high risk of vascular events with aspirin for about 2 years, at a cost of about $20 per patient per year (ie, $40 000 total), prevents about 31 serious vascular events, compared with no aspirin (Box 3) (E1). This equates to spending less than $1250 over 2 years to save one serious vascular event.
Immediate treatment with a 300 mg loading dose of clopidogrel is appropriate for all patients with a suspected non-ST-segment elevation acute coronary syndrome, and for long-term treatment (with 75 mg daily) for at least 9–12 months, in combination with aspirin (E2).7 Clopidogrel is also used, in combination with aspirin in patients undergoing percutaneous coronary intervention and placement of a stent (E2).8,29-31 Clopidogrel is given to patients with ACS scheduled for angiography, unless there is a likelihood that the patients will proceed to surgery within 5 days.
The benefits of clopidogrel, compared with aspirin, in the long-term prevention of serious vascular events in high-risk patients are modest, but significant (odds reduction, 10%; 95% CI, 2%–18%). Treating 1000 patients at high risk of vascular events with clopidogrel for about 2 years, at a cost of about $1095 per patient per year (ie, $2 190 000 in total), prevents about 10 serious vascular events, compared with treatment with aspirin. This equates to spending about $219 000 over 2 years to save one serious vascular event. As the cost to the community of managing a serious vascular event, such as a stroke, is about $50 000, it is not cost-effective to treat all high-risk patients with long-term clopidogrel.32 Clopidogrel should be reserved for patients who are allergic to aspirin, cannot tolerate aspirin, have experienced a recurrent atherothrombotic vascular event while taking aspirin (ie, compliant), or who have an absolute risk of a serious vascular event in excess of 20% per year. In the latter group a 10% relative risk reduction of clopidogrel over aspirin would reduce their risk to 18% per year, which means that treating 100 (or fewer) such high-risk (> 20% per year) patients for 1 year, at a cost of about $100 000 (or less) would prevent two serious vascular events per year (at a cost of about $50 000 each).
The addition of dipyridamole to aspirin for all high-risk patients has not been shown to produce significant additional reductions in serious vascular events (E1).5 However, one large trial in patients with transient ischaemic attack and ischaemic stroke showed substantial reductions in recurrent stroke, but not in myocardial infarction or vascular death.33 Reasons for part or all of the favourable effect on stroke in that study include the possibility that the newer (and more bioavailable) formulation of dipyridamole was more effective than the older preparation used in earlier trials, that dipyridamole reduced stroke by lowering blood pressure rather than an antiplatelet effect, that the comparative dose of aspirin (25 mg twice daily) was insufficient (ie, a placebo), and random error (chance). The combination of dipyridamole and aspirin is being tested further in the European and Australian Stroke Prevention In Reversible Ischaemia Trial (ESPRIT).34
Treatment with a glycoprotein IIb/IIIa receptor inhibitor (together with aspirin and heparin) is recommended in all patients with ACS who undergo percutaneous coronary intervention (E1).9,10 The infusion should be commenced about 24 hours before the procedure and continued for 12 hours (abciximab) or 24 hours (tirofiban, eptifibatide) after the procedure. The benefits are greatest in patients with elevated concentrations of troponin T or I, of whom 11 need to be treated to prevent one death or acute myocardial infarction at 30 days (E1). Patients with ACS who have diabetes also derive particular benefit from glycoprotein IIb/IIIa receptor inhibitors.
Treatment with a glycoprotein IIb/IIIa receptor inhibitor reduces the risk of death or myocardial infarction in patients with non-ST-segment elevation ACS not routinely scheduled for early percutaneous coronary intervention (E1). The event reduction is greatest in patients at high risk of thrombotic complications.9,10
Ongoing trials are examining the safety and effectiveness of abciximab in acute ischaemic stroke (E2).35
Aspirin (75–150 mg/day) decreases the incidence of coronary heart disease in adults who are at increased risk (> 0.6% per year) of heart disease, but it increases the incidence of gastrointestinal bleeding (E1).36-39 For every 1000 patients with a 3% risk of a coronary event over 5 years, long-term aspirin therapy prevents 4–12 coronary events and causes 0–2 haemorrhagic strokes and 2–4 major gastrointestinal bleeding events (E1). This is a benefit-to-harm ratio of about 2.0.
Individuals at increased cardiovascular risk who may wish to consider long-term aspirin therapy (with 75–150 mg/day) are men older than 40 years of age, postmenopausal women, and younger people with risk factors for cardiovascular disease.36-39 Risk factors for cardiovascular disease include increasing age, male sex, cigarette smoking, increasing blood pressure, increasing blood total cholesterol concentration, decreasing high-density lipoprotein cholesterol concentration, raised fasting blood glucose concentration (ie, diabetes mellitus), and a positive family history of cardiovascular disease (in younger adults).36-39
Risk factors for haemorrhagic complications of aspirin include increasing age, any bleeding diathesis, uncontrolled hypertension, and concomitant use of other non-steroidal anti-inflammatory agents or anticoagulants. Enteric-coated or buffered preparations of aspirin do not clearly reduce adverse haemorrhagic effects.
Antiplatelet therapy for high-risk patients reduces the odds of deep-vein thrombosis by 37% (95% CI, 29%–44%) and fatal or non-fatal pulmonary embolism by 53% (95% CI, 41%–63%) among surgical and medical patients.40,41 However, anticoagulants such as heparin or low-molecular-weight heparin are still the preferred method of thromboprophylaxis in most patients, because they are likely to be more effective than aspirin.
1: Pivotal role of platelets in thrombosis and the sites of action of currently approved antiplatelet drugs
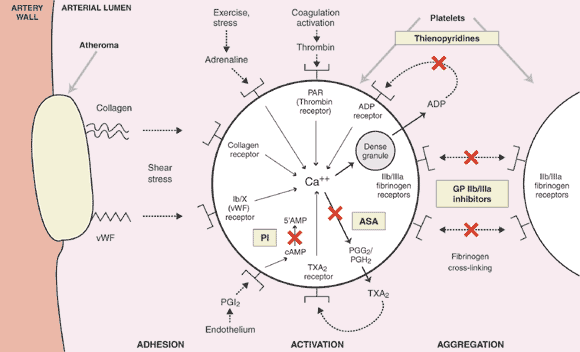
Adhesion of platelets to proteins (collagen, von Willebrand factor), particularly under conditions of high shear stress, and the action of platelet agonists (adrenaline, thrombin, ADP, thromboxane A2) leads to the mobilisation of calcium ion (Ca++), which functions as a mediator of platelet activation. Aspirin inhibits thromboxane A2 synthesis by irreversibly acetylating cyclooxygenase-1; the thienopyridines (clopidogrel, ticlopidine) irreversibly block the ADP receptor; and glycoprotein IIb/IIIa inhibitors block the final common pathway of platelet activation leading to fibrinogen cross-linking of platelets and platelet aggregation. Phosphodiesterase inhibitors (dipyridamole, cilostazol) elevate intracellular cyclic AMP levels and thereby inhibit platelet function.
ADP = adenosine diphosphate; ASA = aspirin; cAMP = cyclic adenosine monophosphate; GP = glycoprotein; PGG2 = prostaglandin G2; PAR = Protease activated receptor; PGH2 = prostaglandin H2; PGI2 = prostacyclin; PI = phosphodiesterase inhibitor; TXA2 = thromboxane A2; vWF = von Willebrand factor.
- Graeme J Hankey1
- John W Eikelboom2
- Royal Perth Hospital and School of Medicine and Pharmacology, University of Western Australia, Perth, WA.
- 1. Badimon L, Badimon J-J, Fuster V. Pathophysiology of arterial thrombosis. In: Gresele P, Page C, Fuster V, Vermylen J, editors. Platelets in thrombotic and non-thrombotic disorders. Cambridge: Cambridge University Press, 2002: 727-737.
- 2. Schedule of pharmaceutical benefits for approved pharmacists and medical practitioners. Canberra: Commonwealth Department of Health and Ageing, November 2002: 94-95.
- 3. Patrono C. Aspirin as an antiplatelet drug. N Engl J Med 1994; 330: 1287-1293.
- 4. Awtry EH, Loscalzo J. Aspirin. Circulation 2000; 101: 1206-1218.
- 5. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. Antithrombotic Trialists' Collaboration. BMJ 2002; 324: 71-86.
- 6. Gent M, Beaumont D, Blanchard J, et al. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348: 1329-1338.
- 7. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. The Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) Trial Investigators. N Engl J Med 2001; 345: 494-502.
- 8. Steinhubl SR, Berger PB, Mann JT III, et al, for the CREDO Investigators. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention. A randomized controlled trial. JAMA 2002; 288: 2411-2420.
- 9. Boersma E, Harrington RA, Moliterno DJ, et al. Platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: a meta-analysis of all major randomised clinical trials. Lancet 2002; 359: 189-198.
- 10. Karvouni E, Katritsis DG, Ioannidis JPA. Intravenous glycoprotein IIb/IIIa receptor antagonists reduce mortality after percutaneous coronary interventions. J Am Coll Cardiol 2003; 41: 26-32.
- 11. National Health and Medical Research Council. A guide to the development, implementation and evaluation of clinical practice guidelines. Canberra: NHMRC, AusInfo, 1999.
- 12. Roderick PJ, Wilkes HC, Meade TW. The gastrointestinal toxicity of aspirin: an overview of randomised controlled trials. Br J Clin Pharmacol 1993; 35: 219-226.
- 13. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry 1991; 54: 1044-1054.
- 14. Taylor DW, Barnett HJM, Haynes RB, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. Lancet 1999; 353: 2179-2184.
- 15. A comparison of two doses of aspirin (30 mg vs 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. Dutch TIA Trial Study Group. N Engl J Med 1991; 325: 1261-1266.
- 16. Derry S, Loke YK. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. BMJ 2000; 321: 1183-1187.
- 17. He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke. A meta-analysis of randomised controlled trials. JAMA 1998; 280: 1930-1935.
- 18. Savcic M, Hauert J, Bachmann F, et al. Clopidogrel loading dose regimens: kinetic profile of pharmacodynamic responses in healthy subjects. Semin Thromb Haemost 1999; 25: 15-19.
- 19. Helft G, Osende JI, Worthley SG, et al. Acute antithrombotic effect of clopidogrel in patients with atherosclerosis on aspirin. Arterioscler Thromb Vasc Biol 2000; 20: 2316-2321.
- 20. Patrono C, Coller B, Dalen JE, et al. Platelet-active drugs: the relationship among dose, effectiveness and side effects. Chest 2001; 119: 39S-63S.
- 21. Hankey GJ, Sudlow CLM, Dunbabin DW. Thienopyridines or aspirin to prevent stroke and other serious vascular events in patients at high risk of vascular disease. Stroke 2000; 31: 1779-1784.
- 22. Moloney BA. An analysis of the side effects of ticlopidine. In: Hass WK, Easton JD, editors. Ticlopidine, platelets and vascular disease. New York: Springer, 1993: 117-139.
- 23. Bennett CL, Davidson CJ, Raisch DW, et al. Thrombotic thrombocytopenic purpura associated with ticlopidine in the setting of coronary artery stents and stroke prevention. Arch Intern Med 1999; 159: 2524-2528.
- 24. Bennett CL, Connors JM, Carwile JM, et al. Thrombotic thrombocytopenic purpura associated with clopidogrel. N Engl J Med 2000; 342: 1773-1777.
- 25. Bennett CL, Connors JM, Moake JL. Clopidogrel and thrombotic thrombocytopenic purpura. N Engl J Med 2000; 343: 1191-1194.
- 26. Hankey GJ. Clopidogrel and thrombotic thrombocytopenic purpura. Lancet 2000; 356: 269-270.
- 27. Curtis BR, Swyers J, Divgi A, et al. Thrombocytopenia after second exposure to abciximab is caused by antibodies that recognize abciximab-coated platelets. Blood 2002; 99: 2054-2059.
- 28. Hankey GJ on behalf of the National Blood Pressure Advisory Committee of the National Heart Foundation. Non-valvular atrial fibrillation and stroke prevention. Med J Aust 2001; 174: 234-348. <eMJA full text>
- 29. Müller C, Büttner HJ, Petersen J, Roskamm H. A randomized comparison of clopidogrel and aspirin versus ticlopidine and aspirin after the placement of coronary-artery stents. Circulation 2000; 101: 590-593.
- 30. Bertrand ME, Rupprecht H-J, Urban P, Gershlick AH, for the CLASSICS Investigators. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting. The Clopidogrel Aspirin Stent International Cooperative Study (CLASSICS). Circulation 2000; 102: 624-629.
- 31. Mehta SR, Yusuf S, Peters RJG, et al, for the Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358: 527-533.
- 32. Gaspoz J-M, Coxson PG, Goldman PA, et al. Cost-effectiveness of aspirin, clopidogrel, or both for secondary prevention of coronary heart disease. N Engl J Med 2002; 346: 1800-1806.
- 33. Diener HC, Cunha L, Forbes C, et al. European stroke prevention study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci 1996; 143: 1-13.
- 34. De Schryver ELLM, on behalf of the European/Australian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT) Group. Design of ESPRIT: an international randomised trial for secondary prevention after non-disabling cerebral ischaemia of arterial origin. Cerebrovasc Dis 2000; 10: 147-150.
- 35. Abciximab in acute ischemic stroke. A randomised, double-blind, placebo-controlled, dose-escalation study. The Abciximab in Ischemic Stroke Investigators. Stroke 2000; 31: 601-609.
- 36. US Preventive Services Task Force. Aspirin for the primary prevention of cardiovascular events: recommendation and rationale. Ann Intern Med 2002; 136: 157-160.
- 37. Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med 2002; 136: 161-172.
- 38. Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for primary prevention of cardiovascular disease and stroke: 2002 update. Consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. Circulation 2002; 106: 388-391.
- 39. Lauer MS. Aspirin for primary prevention of coronary events. N Engl J Med 2002; 346: 1468-1474.
- 40. Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy-III: Reduction in venous thromboembolism and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients. BMJ 1994; 308: 235-246.
- 41. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin. Pulmonary Embolism Prevention (PEP) Trial. Lancet 2000; 355: 1295-1302.





Abstract
Antiplatelet drugs protect against myocardial infarction, stroke, cardiovascular death and other serious vascular events in patients with a history of previous vascular events or known risk factors for cardiovascular disease.
Aspirin reduces the risk of serious vascular events in patients at high risk of such an event by about a quarter and is recommended as the first-line antiplatelet drug.
Clopidogrel reduces the risk of serious vascular events among high-risk patients by about 10% compared with aspirin. It is as safe as aspirin, but much more expensive. It is an appropriate alternative to aspirin for long-term secondary prevention in patients who cannot tolerate aspirin, have experienced a recurrent vascular event while taking aspirin, or are at very high risk of a vascular event (≥ 20% per year).
Addition of clopidogrel to aspirin reduces the risk of serious vascular events among patients with non-ST-segment elevation acute coronary syndromes by 20%, and patients undergoing percutaneous coronary intervention by 30%, compared with aspirin alone.
Addition of a glycoprotein IIb/IIIa receptor antagonist to aspirin reduces the risk of vascular events among patients with non-ST-segment elevation acute coronary syndromes by 10% and among patients undergoing percutaneous coronary intervention by 30%, compared with aspirin alone; it appears to provide incremental benefit in patients also treated with clopidogrel.
Addition of dipyridamole to aspirin seems to be more effective than aspirin alone for preventing recurrent stroke, but its overall effect in preventing serious vascular events in patients with ischaemic stroke and transient ischaemic attack has not been determined.