Considerable controversy surrounds the diagnosis,1 natural history, management and outcomes2 of women whose initial diagnosis of glucose intolerance occurs during pregnancy (ie, gestational diabetes mellitus [GDM]). Opinions regarding the impact on the infant range from only minor to highly significant.3 The true incidence of GDM is unknown because of the lack of universal testing, inconsistent diagnostic criteria and the absence of a uniform reporting system to a centralised register. Some of this uncertainty arises from the paucity of true population studies.3,4
In Australia, previous attempts to determine the true incidence have been based on surveys of prenatal clinics consisting of hospital patients and/or private patients,5-8 but these are subject to bias due to referral patterns, local population variations and local clinical practice.
Using information from routinely collected datasets and epidemiological methods, we aimed to estimate the incidence of GDM in all singleton pregnancies for Victoria in 1996 and compare the risk factors for GDM and the maternal and infant outcomes with those in non-diabetic pregnancies.
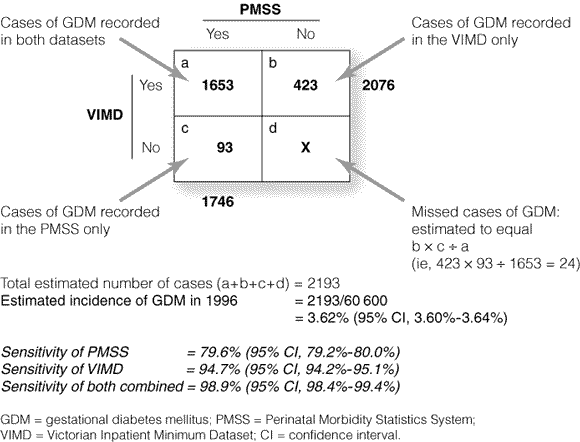
Our study used data from the Perinatal Morbidity Statistics System (PMSS) and the Victorian Inpatient Minimum Dataset (VIMD). Linking independent data sources and applying capture–recapture methodology can provide an estimate of the level of incompleteness of a dataset.9 Having identified the cases reported to one dataset only and those reported to both, one can estimate the number of unreported cases and ultimately the total number of cases (Box 1).
After excluding women who had multiple births, an estimate of the total number of cases, their incidence and the 95% confidence intervals (95% CI), and the sensitivity of the datasets were calculated in Excel10 using Chapman and Seber's calculations for the capture–recapture, as quoted in Epi Info version 6.04a.11
In the absence of a unique identifier, a simple merging of the files was not feasible. Probabilistic record linkage using computer software (AutoMatch)12 was used to link maternal records from the PMSS with the corresponding record in the VIMD. Reporting of births by midwives to the PMSS is mandatory in Victoria, with compliance at 99.6% of all births.13 Variables common to both datasets were used in various combinations to identify matches. The programming of the software was piloted using small datasets until there were no obviously incorrect matches, few unlinked records, and a minimum number of records for individual review.
Ethical approval to perform record linkage between the two departmental datasets was obtained from the Victorian Department of Human Services Ethics Committee.
Since the capture–recapture methods estimate the number of unascertained cases of GDM, these cases are hypothetical and cannot be assigned to specific records. Therefore, the cases of GDM used in the analysis of risk factors and outcomes were those identified in either or both datasets. Two hundred women with pre-existing diabetes were excluded from the multivariate analysis.
Factors recorded in the PMSS that were investigated for their association with GDM included maternal age, country of birth, history of a previous perinatal death, aboriginality, parity, gravidity, marital status, rural or metropolitan area of residence, and the size of the hospital where the birth occurred.
The outcomes investigated were those recorded in the perinatal data collection form, either in "tick boxes" (eg, placenta praevia, placental abruption, onset of labour and delivery type, neonatal death), or coded as a complication (eg, hypertension [International Classification of Diseases, 9th edition (ICD-9), codes 6420 to 6429], or pre-eclampsia [ICD-9 code 6424]). Macrosomia was defined as birthweight ≥ 90th percentile for gestational age, and birth defect information was merged from the Congenital Malformation Registry. Factors other than a diagnosis of GDM that are known to influence the development of these outcomes were included in the analysis. These included maternal age, marital status, country of birth, gravidity and parity, aboriginality, previous perinatal death, maternal hypertension or pre-eclampsia, gestational age, baby's discharge status, stillbirth, gender, and macrosomia.
Univariate and multivariate statistical analysis was performed using Epi Info 6.04a11 or SPSS.12 We calculated χ2 tests, χ2 tests for trend and odds ratio (an approximation to the relative risk for a condition with an incidence < 10%) for all risk factors and outcomes (significance defined as P < 0.05). We performed a forward stepwise logistic regression analysis of the explanatory variables to assess the independent effects on the odds of developing GDM. The effects of gravidity and parity were very similar, so only parity is reported here. Similarly, adverse outcomes that were significantly associated with GDM in the χ2 test and those considered clinically important were modelled as the dependent variable in a forward stepwise logistic regression to identify the extent to which the diagnosis of GDM increased the risk of that outcome after controlling for other predictors. While we have reported the 95% CI, because of the multiple comparisons we have reduced the level considered significant to P < 0.01.
We could link records from the two datasets for 99.3% of the 61 912 women giving birth in Victoria in 1996. After excluding women who had multiple births, there were 60 600 in our linked study population. Box 1 shows the number of women who were diagnosed with GDM in one or both datasets. Using capture–recapture methodology, the estimated number not identified in any dataset was 24 and the total number of cases of GDM in 1996 was 2193. This corresponds to 3.62% (95% CI, 3.60%–3.64%) of all women having singleton births.
Factors significantly associated with GDM (P < 0.001) were the woman's country of birth, marital status and rural residence, maternal age, parity, gravidity, and index of relative social disadvantage (the only inverse relationship). Previous perinatal death was associated with GDM at the P = 0.015 level. Aboriginality was not significantly associated. The incidence of GDM was usually higher in women who were born overseas, particularly northeast or southern Asia, and was lowest in women born in Australia or the United Kingdom.
Logistic regression analysis showed that, after controlling for other factors, the factors associated with the diagnosis of GDM were having been born overseas (other than in the British Isles, North or South America), aboriginality, being older, residing outside the metropolitan region, living in a lower socio-economic status region, and giving birth in a larger hospital. Parity, marital status and previous perinatal death were not significantly associated with GDM. The crude incidence of GDM was higher in the metropolitan region than in the rural region, but, after adjustment for the other variables, residing in a rural region contributed to an increased risk of being diagnosed with GDM. The strongest associations with adjusted odds ratios greater than two were for some ethnic groups, women over 35 years of age and Aboriginal women.
Maternal outcomes: Rates of hypertension and pre-eclampsia were increased in women with GDM. "Hypertension" included pre-existing hypertension as well as hypertensive disorders of pregnancy. This association stayed after controlling for parity, country of birth and maternal age, which were all strongly associated with the development of hypertension and pre-eclampsia. Placenta praevia, placental abruption and premature rupture of membranes were not increased in women with GDM.
Delivery outcomes: The intervention rate was higher in women with GDM. They were more likely to have labour induced or have an elective caesarean and to undergo an operative delivery (forceps or caesarean). Those who had a vaginal birth were more likely to have an episiotomy (even after controlling for the effect of forceps delivery). After controlling for the presence of hypertension and pre-eclampsia the risk of having labour induced was even higher.
Fetal outcomes: Infants of mothers with GDM were more likely to have hyaline membrane disease, neonatal jaundice and macrosomia. These findings were present after controlling for gestational age and prematurity. More severe perinatal outcomes, such as stillbirth, neonatal death and shoulder dystocia, were not associated with GDM.
This article demonstrates the usefulness of record linkage using computer software and capture–recapture methodology in estimating the incidence of conditions at the population level. The combination of information from the two data sources has compensated for the under-reporting of this condition in the individual datasets, and we are therefore able to count 99% of diagnosed cases of GDM in Victoria.
The estimated incidence of gestational diabetes for 1996 of 3.6% is very close to the average of 3.3% estimated for New South Wales for 1991 to 1994,14 despite the different methods used to determine the level of under-reporting
In 1991, and again in 1998, the Australian Diabetes in Pregnancy Society guidelines recommended that all women should be screened for GDM.15,16 Until there is universal screening, the percentage of pregnant women diagnosed as having GDM will underestimate the incidence. Previous reports have suggested that in the early 1990s only 50% or less of pregnant women were screened for GDM.14 However, the women who are tested are more likely to be in the higher-risk groups, so the incidence might be lower in the untested, undiagnosed group.
Previous estimates of GDM incidence worldwide vary from 1% to 10%. Previous Australian reports may have over-estimated the incidence.5-8 Our population-based study showed that the incidence was higher in women attending the larger hospitals or living in the metropolitan regions — sites where earlier studies have been conducted.
A limitation of this study is the lack of information on some risk factors for GDM, such as previous history of GDM, previous birth of a large-for-gestational-age baby, a family history of type 2 diabetes and the woman's body mass index before conception. However, we have been able to examine in a logistic regression many other potential risk factors at a population level.
In Victoria, about 25% of the women giving birth in any one year were not born in Australia. Almost all of the non-Australian-born groups have a higher frequency of GDM. These groups also tend to be older when they give birth,17 which may further contribute to the higher frequency. Generally, the incidence of GDM in different racial groups reflects their propensity for developing type 2 diabetes.7
The link between gestational diabetes and poor maternal and fetal outcomes has been recognised for a long time.3,4,14,15,18,19 The increased risk of hypertension and pre-eclampsia was a significant finding and is consistent with previous reports.4,20-23 Women with GDM have been described as fitting into the "metabolic syndrome" because of their tendency to increased body mass index, higher serum triglyceride and free fatty acid levels and insulin resistance,24 and this may partially explain the link with hypertension and pre-eclampsia.
Women with diagnosed GDM had a much higher risk of induced delivery (37.3% v 22.7%). This has been reported previously overseas20,23 and locally,25 and is ascribed to the perceived increased risk of macrosomia, shoulder dystocia and delivery complications in these women.
The increased odds ratio for caesarean section is supported by numerous studies,3,4,20-23,26,27 and occurred after adjustment for the presence of macrosomia. It has been noted for some time that the link between GDM and interventional outcomes may become a self-fulfilling prophecy, where the decision to intervene may be influenced by the diagnosis of GDM itself rather than other indications.28 We cannot be sure if this was happening in Victoria in 1996 without linking birthweight data and glucose levels with the caesarean outcome. Some local centres have not found an increased rate of caesarean section in GDM pregnancies.29
Operative delivery and episiotomy were also more likely in GDM pregnancies — even after adjustment for the higher prevalence of macrosomia in this group.
For the infant, hyaline membrane disease and neonatal jaundice were more common in GDM pregnancies, and this is consistent with overseas experience.30 Macrosomia, whether defined as ≥ 4000 g birthweight or birthweight ≥ 90th centile, is one of the major infant morbidities associated with GDM. Some studies have reported normalised rates of macrosomia and other major neonatal and maternal outcomes with intensive antenatal treatment.31-33 These are predominantly reports from large tertiary centres, where common unit policies and rigorous guidelines may have helped to achieve the outcomes. Our data and other large studies3,4 suggest that, on a population level, women identified as having GDM still have an increased rate of macrosomia.
Recent reports have found increased perinatal mortality associated with GDM.4,23,30 We did not find raised perinatal mortality, but our study did not have the power to detect a difference of the order previously reported.
A strength of this study is the large numbers involved and the fact that it examines an unselected population. Retrospective studies do rely on the accuracy of the recorded data, however, and there is a potential for recorder bias: women who develop complications may be more likely to be identified as having GDM. Their infants are also more likely to be tested for hypoglycaemia and jaundice. In addition, in this analysis, the diagnoses of hypertension, pre-eclampsia, neonatal jaundice and hyaline membrane disease are as recorded in the datasets and are not subject to a single standard definition or validation of entry. Finally, while many centres in Victoria used criteria similar to the Australian Diabetes in Pregnancy Society criteria for diagnosing GDM, they were not adopted by all,34 so our population of women have not all necessarily been subject to the same diagnostic criteria.
There are several different criteria worldwide and the diagnosis of GDM has been subject to much controversy,2 as there is no clear level of glucose above which the maternal and fetal outcomes become distinctly worse. Instead, there seems to be a continuously increasing risk as maternal glucose levels rise.35
The important question that remains to be resolved in the management of GDM is: at what level of hyperglycaemia do the adverse perinatal and maternal outcomes justify treatment and interventions? This cannot be answered on the basis of retrospective observational data, but the ongoing HAPO (Hyperglycaemia and Adverse Pregnancy Outcomes) study, taking place in Australia and around the world, will go some way to address this question.
In our study, women identified as having gestational diabetes had increased rates of hypertension, pre-eclampsia, induced labour, and interventional delivery. Their offspring had a higher risk of macrosomia, neonatal jaundice and hyaline membrane disease. Thus, it does seem important to identify all women with GDM and to continue to monitor their outcomes.
Risk factor |
Total births* |
GDM cases |
Crude odds ratio† (95% CI) |
Adjusted odds ratio†‡ (95% CI) |
|||||||
Aboriginality |
|||||||||||
Non-Aboriginal |
59 962 |
3.6% |
Reference |
Reference |
|||||||
Aboriginal |
438 |
4.3% |
1.2 (0.75–1.97) |
2.5 (1.54–4.06) |
|||||||
Country of birth§ |
|||||||||||
Australia |
45 088 |
2.4% |
Reference |
Reference |
|||||||
Oceania |
1 415 |
4.4% |
1.8 (1.40–2.40) |
1.7 (1.26–2.16) |
|||||||
UK and Eire |
2 451 |
2.9% |
1.2 (0.95–1.54) |
1.0 (0.76–1.26) |
|||||||
Southern Europe |
1 636 |
5.6% |
2.4 (1.91–2.97) |
1.8 (1.47–2.31) |
|||||||
N, W and E Europe |
911 |
5.6% |
2.4 (1.78–3.17) |
2.0 (1.50–2.68) |
|||||||
Middle East |
1 679 |
5.4% |
2.3 (1.82–2.82) |
2.1 (1.65–2.62) |
|||||||
SE Asia |
3 326 |
9.2% |
4.1 (3.56–4.64) |
3.3 (2.82–3.76) |
|||||||
NE Asia |
1 215 |
13.7% |
6.3 (5.32–7.53) |
4.7 (3.92–5.64) |
|||||||
Southern Asia |
1 140 |
12.5% |
5.7 (4.77–6.90) |
4.7 (3.92–5.76) |
|||||||
North America |
294 |
2.0% |
0.8 (0.37–1.87) |
0.7 (0.32–1.64) |
|||||||
South America |
371 |
3.8% |
1.6 (0.92–2.68) |
1.4 (0.80–2.36) |
|||||||
Africa |
743 |
8.2% |
3.6 (2.73–4.68) |
3.1 (2.37–4.10) |
|||||||
At sea/unknown |
131 |
3.8% |
1.6 (0.65–3.89) |
1.6 (0.67–4.04) |
|||||||
Maternal age-group |
|||||||||||
< 20 years |
2 111 |
1.0% |
0.4 (0.26–0.62) |
0.4 (0.29–0.68) |
|||||||
20–24 years |
8 889 |
1.8% |
0.7 (0.61–0.86) |
0.7 (0.59–0.84) |
|||||||
25–29 years |
20 058 |
2.5% |
Reference |
Reference |
|||||||
30–34 years |
19 939 |
4.1% |
1.7 (1.48–1.86) |
1.7 (1.49–1.87) |
|||||||
35–39 years |
8 148 |
6.5% |
2.7 (2.35–3.01) |
2.5 (2.19–2.83) |
|||||||
40–44 years |
1 216 |
9.8% |
4.2 (3.39–5.14) |
3.8 (3.09–4.74) |
|||||||
>45 years |
39 |
12.8% |
5.7 (2.20–14.53) |
5.0 (1.90–13.16) |
|||||||
Region |
|||||||||||
Rural |
16 019 |
2.5% |
0.6 (0.54–0.67) |
1.2 (1.07–1.43) |
|||||||
Metropolitan |
43 782 |
4.0% |
Reference |
Reference |
|||||||
Hospital group |
|||||||||||
< 100 births |
1 778 |
1.1% |
0.2 (0.13–0.32) |
0.3 (0.17–0.43) |
|||||||
100–999 births |
21 335 |
2.4% |
0.5 (0.41–0.51) |
0.6 (0.50–0.66) |
|||||||
1000–1999 births |
13 906 |
3.3% |
0.6 (0.57–0.71) |
0.8 (0.68–0.86) |
|||||||
> 2000 births |
23 381 |
5.1% |
Reference |
Reference |
|||||||
IRSED** |
|||||||||||
Lowest quintile |
11 910 |
4.7% |
Reference |
Reference |
|||||||
Lower quintile |
11 895 |
3.2% |
0.7 (0.59–0.77) |
1.0 (0.87–1.16) |
|||||||
Average quintile |
11 901 |
3.1% |
0.7 (0.57–0.75) |
1.0 (0.89–1.19) |
|||||||
Higher quintile |
11 912 |
3.6% |
0.8 (0.67–0.87) |
1.0 (0.87–1.14) |
|||||||
Highest quintile |
11 929 |
3.4% |
0.7 (0.62–0.81) |
0.8 (0.70–0.94) |
|||||||
*The sum of the total number of births in each category for each risk factor does not always equal 60 400, as a small percentage may be in the "unknown" category. †Approximates to relative risk for low-incidence conditions, significant at the P = 0.05 level if the 95% CI does not include 1.0. ‡Adjusted for aboriginality, country of birth, age, region, delivery hospital, socioeconomic status, marital status, previous perinatal death and either gravidity or parity in logistic regression using the forward stepwise option in SPSS.12 §Country groupings are based on Australian Bureau of Statistics definitions. The countries that are the major contributors to our population are specified. Southern Europe: Greece, Italy and countries of the former Yugoslavia. Middle East: Lebanon, Turkey and Cyprus. SE Asia: Vietnam, Philippines, Malaysia, Cambodia, Indonesia. NE Asia: China, Hong Kong. Southern Asia: India, Sri Lanka. **IRSED = Index of Relative SocioEconomic Disadvantage, Australian Bureau of Statistics. |
|||||||||||
Risk factor |
Total births* |
GDM cases |
Crude odds ratio† (95% CI) |
||||||||
Parity |
|||||||||||
None |
24 055 |
3.3% |
Reference |
||||||||
One |
21 316 |
3.6% |
1.1 (0.99–1.21) |
||||||||
Two |
9 961 |
3.9% |
1.2 (1.04–1.34) |
||||||||
Three or more |
5 068 |
4.5% |
1.4 (1.19–1.61) |
||||||||
Previous perinatal death |
|||||||||||
None |
59 351 |
3.6% |
Reference |
||||||||
One or more |
1 049 |
5.1% |
1.4 (1.01–1.90) |
||||||||
*The sum of the births in each category for each risk factor does not always equal 60 400, as a small percentage may be in the "unknown" category. †Approximates to relative risk for low-incidence conditions, significant at the P = 0.05 level if the 95% CI does not include 1.0. |
|||||||||||
4: Adverse outcomes in pregnancies affected by gestational diabetes
Outcome |
Incidence in non-diabetic women (n = 58 231) |
Incidence in women with GDM (n = 2169) |
Crude relative risk (95% CI) |
Adjusted odds ratio (95% CI)† |
|||||||
Hypertension |
7.6% |
11.6%* |
1.5 (1.36–1.72) |
1.6 (1.4–1.9) |
|||||||
Pre-eclampsia |
5.2% |
8.1%* |
1.6 (1.34–1.79) |
1.6 (1.4–1.9) |
|||||||
Placenta praevia |
0.8% |
1.0% |
1.3 (0.86–2.01) |
NS |
|||||||
Placental abruption |
0.7% |
0.5% |
0.8 (0.41–1.37) |
NS |
|||||||
Premature rupture of membranes |
4.8% |
5.0% |
1.1 (0.88–1.27) |
NS |
|||||||
Induced labour |
22.7% |
37.3%* |
1.8 (1.74–1.93) |
3.0 (2.7–3.4) |
|||||||
No labour |
10.6% |
20.1% |
2.3 (2.14–2.52) |
2.5 (2.2–2.9) |
|||||||
Episiotomy (vaginal births, n = 48 802) |
22.5% |
29.9%* |
1.3 (1.23–1.44) |
1.2 (1.1–1.4) |
|||||||
Elective caesarean |
10.6% |
20.1%* |
1.9 (1.70–2.10) |
1.9 (1.7–2.2) |
|||||||
Emergency caesarean |
8.1% |
11.8% |
1.5 (1.30–1.60) |
1.5 (1.3–1.7) |
|||||||
Caesarean total |
18.7% |
31.9%* |
1.7 (1.60–1.82) |
1.7 (1.6–1.9) |
|||||||
Operative delivery (forceps and caesarean) |
28.7% |
41.0%* |
1.4 (1.36–1.51) |
1.5 (1.4–1.7) |
|||||||
Hyaline membrane disease/respiratory distress syndrome |
1.5% |
2.5%* |
1.7 (1.31–2.25) |
1.6 (1.2–2.2) |
|||||||
Neonatal jaundice |
7.4% |
12.8%* |
1.7 (1.53–1.93) |
1.4 (1.2–1.7) |
|||||||
Macrosomia |
9.7% |
16.7%* |
1.7 (1.56–1.89) |
2.0 (1.8–2.3) |
|||||||
Shoulder dystocia |
1.0% |
1.2% |
1.3 (0.88–1.89) |
NS |
|||||||
Stillbirth |
0.6% |
0.3% |
0.5 (0.24–1.07) |
NS |
|||||||
Neonatal death |
0.4% |
0.2% |
0.6 (0.25–1.47) |
NS |
|||||||
Congenital malformation |
2.9% |
3.1% |
1.1 (0.84–1.35) |
NS |
|||||||
* χ2 test; P < 0.005. † All adjusted odds ratios are significant to P < 0.001 level, except episiotomy (P = 0.004) and hyaline membrane disease (P = 0.002). All odds ratios were adjusted for maternal country of birth, aboriginality, marital status, age, gestation, parity, socioeconomic status based on postcode, previous perinatal death, and congenital malformation in current pregnancy. When they were not the outcome of interest, adjustments were made for macrosomia, hypertension or pre-eclampsia (hypertension or pre-eclampsia in separate models – note hypertension includes pre-eclampsia). In all analyses except the delivery and labour outcome we also adjusted for the sex of the child and the birth condition. NS = not significant. GDM = gestational diabetes mellitus. |
|||||||||||
Received 13 November 2001, accepted 4 July 2002
- Christine A Stone1
- Kylie A McLachlan2
- Jane L Halliday3
- Peter Wein4
- Christine Tippett5
- 1 Prevention and National Health Priorities, Public Health, Department of Human Services, Melbourne, VIC.
- 2 Department of Endocrinology and Diabetes, St Vincent's Hospital, Fitzroy, VIC.
- 3 Birth Defect Registry, Perinatal Data Collection Unit, Public Health, Department of Human Services, Melbourne, VIC.
- 4 Department of Obstetrics and Gynaecology, University of Melbourne, East Melbourne, VIC.
- 5 Maternal Fetal Medicine, Monash Medical Centre, Clayton, VIC.
We thank Associate Professor Frank Alford, Professor Shaun Brennecke and Professor Norman Beischer for reading the manuscript and for discussions. We would like to acknowledge the midwives who completed the Perinatal Data Collection Unit forms, the staff of the Perinatal Data Collection Unit who processed the forms, the members of the Consultative Council on Obstetric and Paediatric Mortality and Morbidity who oversaw the collection, the health information managers who extracted the inpatient data, and the Victorian women who were the subjects of this report.
- 1. Kjos S, Buchanan T. Gestational diabetes mellitus. N Engl J Med 1999; 341: 1749-1755.
- 2. Khandelwal M, Homko C, Reece A. Gestational diabetes mellitus: controversies and current opinions. Curr Opin Obstet Gynecol 1999; 11: 157-165.
- 3. Aberg A, Rydhstroem H, Frid A. Impaired glucose tolerance associated with adverse pregnancy outcome: a population based study in southern Sweden. Am J Obstet Gynecol 2001; 184: 77-83.
- 4. McMahon M, Ananth C, Liston R. Gestational diabetes mellitus: risk factors, obstetric complications and infant outcomes. J Reprod Med 1998; 43: 372-378.
- 5. Beischer N, Wein P, Sheedy M, et al. Identification and treatment of women with hyperglycaemia diagnosed during pregnancy can significantly reduce perinatal mortality rates. Aust N Z J Obstet Gynaecol 1996; 36: 239-247.
- 6. Moses RG, Griffiths RD, McPherson S. The incidence of gestational diabetes mellitus in the Illawarra area of New South Wales. Aust N Z J Obstet Gynaecol 1994; 34: 425-427.
- 7. Yue D, Molyneaux L, Ross G. Why does ethnicity affect the prevalence of gestational diabetes? The underwater volcano theory. Diabet Med 1996; 13: 748-752.
- 8. Martin FI, Ratnaike S, Wootton A, et al. The 75 g oral glucose tolerance test in pregnancy. Diabetes Res Clin Pract 1995; 27: 147-151.
- 9. Hook EB, Regal RR. Capture–recapture methods in epidemiology: methods and limitations. Epidemiol Rev 1995; 17: 243-264.
- 10. Microsoft Corporation. Excel for Windows 95 [computer program].
- 11. Epi Info version 6.04a [computer program]. Geneva, Switzerland: Centers for Disease Control and Prevention (CDC) USA and World Health Organization.
- 12. Automatch. In: SPSS for Windows version 8.0 [computer program]. Chicago, Illinois: SPSS Inc.
- 13. Riley M, Griffin O. Validating a statewide data collection: differences in information technology resources between hospitals. J Health Info Manage 1997; 27(2): 67-68.
- 14. Moses RG, Colagiuri S. The extent of undiagnosed gestational diabetes mellitus in New South Wales. Med J Aust 1997; 167: 14-16. <eMJA full text>
- 15. Hoffman L, Nolan C, Wilson JD, et al. Gestational diabetes mellitus — management guidelines. The Australasian Diabetes in Pregnancy Society. Med J Aust 1998; 169: 93-97. <eMJA full text>
- 16. Martin FI. The diagnosis of gestational diabetes. Ad Hoc Working Party. Med J Aust 1991; 155: 112.
- 17. Riley M, Halliday J. Births in Victoria 1992–1996. Melbourne: Perinatal Data Collection Unit, Department of Human Services; 1998.
- 18. Kjos S, Buchanan T. Gestational Diabetes — risk or myth? J Clin Endocrinol Metab 1999; 84: 1854-1857.
- 19. Dandrow R, O'Sullivan J. Obstetric hazards of gestational diabetes. Am J Obstet Gynecol 1966; 96: 1144-1147.
- 20. Jensen DM, Sorensen B, Feilberg-Jorgensen N, et al. Maternal and perinatal outcomes in 143 Danish women with gestational diabetes mellitus and 143 controls with a similar risk profile. Diabet Med 2000; 17: 281-286.
- 21. Sermer M, Naylor CD, Farine D, et al. The Toronto Tri-Hospital Gestational Diabetes Project. A preliminary review. Diabetes Care 1998; 21 Suppl 2: B33-B42.
- 22. Wen SW, Liu S, Kramer MS, et al. Impact of prenatal glucose screening on the diagnosis of gestational diabetes and on pregnancy outcomes. Am J Epidemiol 2000; 152(11): 1009-1014; discussion 1015-1016.
- 23. Casey BM, Lucas MJ, McIntire DD, et al. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstet Gynecol 1997; 90: 869-873.
- 24. Clark C, Qui C, Amerman BEA. Gestational diabetes: Should it be added to the syndrome of insulin resistance? Diabetes Care 1997; 20: 867-871.
- 25. Simpson R, Kast S. Management of gestational diabetes with a conservative insulin protocol. Med J Aust 2000; 172: 537-540.
- 26. Sugaya A, Sugiyama T, Nagata M, et al. Comparison of the validity of the criteria for gestational diabetes mellitus by WHO and by the Japan Society of Obstetrics and Gynecology by the outcomes of pregnancy. Diabetes Res Clin Pract 2000; 50: 57-63.
- 27. Magee MS, Walden CE, Benedetti TJ, et al. Influence of diagnostic criteria on the incidence of gestational diabetes and perinatal morbidity. JAMA 1993; 269: 609-615.
- 28. Coustan DR. Management of gestational diabetes mellitus: a self-fulfilling prophecy? JAMA 1996; 275: 1199-1200.
- 29. Moses R. Gestational diabetes: is a higher caesarean section rate inevitable? Diabetes Care 2000; 23: 15-17.
- 30. Persson B, Hanson U. Neonatal morbidities in gestational diabetes mellitus. Diabetes Care 1998; 21 Suppl 2: B79-B84.
- 31. Thompson DM, Dansereau J, Creed M, Feingold L. Tight glucose control results in normal perinatal outcome in 150 patients with gestational diabetes. Obstet Gynecol 1994; 83: 362-366.
- 32. Langer O, Rodriguez DA, Xenakis EM, et al. Intensified versus conventional management of gestational diabetes. Am J Obstet Gynecol 1994; 170: 1036-1046; discussion 1046-1047.
- 33. Drexel H, Bichler A, Sailer S, et al. Prevention of perinatal morbidity by tight metabolic control in gestational diabetes mellitus. Diabetes Care 1988; 11: 761-768.
- 34. Rumbold AR, Crowther CA. Guideline use for gestational diabetes mellitus and current screening, diagnostic and management practices in Australian hospitals. Aust N Z J Obstet Gynaecol 2001; 41: 86-90.
- 35. Moses RG, Calvert D. Pregnancy outcomes in women without gestational diabetes mellitus related to the maternal glucose level. Is there a continuum of risk? Diabetes Care 1995; 18: 1527-1533.





Abstract
Objectives: To describe the epidemiology of gestational diabetes mellitus (GDM) in Victoria.
Study design: Population study of all women having singleton births in Victoria in 1996.
Methods: Probabilistic record linkage of routinely collected data and capture–recapture techniques to provide an estimate of the incidence of GDM.
Main outcome measures: Risk factors for and the adverse outcomes associated with GDM compared with the non-diabetic population by univariate and multivariate analysis.
Results: The estimated incidence of GDM was 3.6% (95% confidence interval [CI], 3.60%–3.64%). GDM is associated with women who are older, Aboriginal, non-Australian born, or who give birth in a larger hospital. The adverse outcomes associated with GDM pregnancies were hypertension/pre-eclampsia (adjusted odds ratio [OR], 1.6; 95% CI, 1.4–1.9), hyaline membrane disease (1.6; 1.2–2.2), neonatal jaundice (1.4; 1.2–1.7) and macrosomia (2.0; 1.8–2.3). Interventions during childbirth were also associated with GDM — for example, induction of labour (3.0; 2.7–3.4) and caesarean section (1.7; 1.6–1.9).
Conclusion: Women with GDM had increased rates of hypertension, pre-eclampsia, induced labour, and interventional delivery. Their offspring had a higher risk of macrosomia, neonatal jaundice and hyaline membrane disease.