In the late 1990s, nine coordinated care trials took place across Australia. Their overall aim was to test whether multidisciplinary care planning and service coordination improved health and wellbeing for people with chronic health conditions or complex care needs within existing resources.1 A recent review of studies of outreach nursing, which has features in common with coordinated care, found increased costs and no reduction in hospitalisation, but slight improvements in quality of life.2
In the South Australian trial, HealthPlus,3 one of eight components was the Western Respiratory Project. Its aims included:
sharing of care between hospital-based specialists, general practitioners, and other community-based healthcare practitioners (including domiciliary care, Royal District Nursing Service) in a community-based approach, with GPs central to the planning and monitoring of prospective care;
pooling of federal and state health funds to provide efficient healthcare service delivery and enhance patient care with similar or reduced overall healthcare system costs; and
producing evidence on the change in health outcomes as a result of the changes in healthcare delivery.
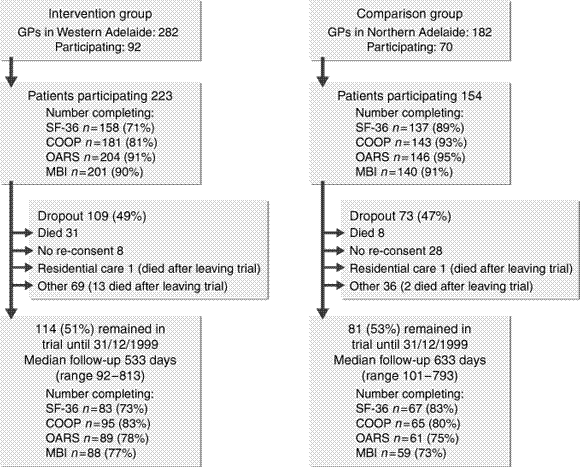
Recruitment began in July 1997 and continued until September 1998. Follow-up ended in December 1999. Patients were recruited from the western (intervention group) and northern (comparison group) suburbs of Adelaide, in collaboration with the Adelaide western and northern Divisions of General Practice. The geographical comparison region was selected pre-hoc on the basis of previous surveys4 as the region of Adelaide that best matches the sociodemographic features of the western region. The number of GPs per 1000 patients (northern, 1.21; western, 1.34) is also similar for the two regions. Participating GPs recruited patients opportunistically, so details of the eligible population were not collected.
This study should be considered as a form of "action research", where a cycle of action and critical review led to improved GP participation and refinement of the methodology throughout the study.5,6
Ethics approval was obtained by the Ethics of Human Research Committee at the North Western Adelaide Health Service and clearance to obtain data was received from the Health Insurance Commission.
GPs took the role of "care coordinators" and supervised the multidisciplinary management of each patient.7 To facilitate this process, multidisciplinary care plan generators (CPGs) were constructed with input from consumers (patients and their carers), GPs, respiratory physicians, allied health professionals, the Royal District Nursing Service, domiciliary care and an epidemiologist. Relevant published medical evidence and the published guidelines of the thoracic societies of Australia and New Zealand, Britain and America8-10 were incorporated. The CPGs included a recommended annual number of GP visits, respiratory function tests, other diagnostic tests and physician visits where necessary.
GPs received and were reimbursed for two to four hours of orientation.
GPs were supported by "service coordinators" (nurses), who liaised with the patient, GP, respiratory specialist and other healthcare professionals, monitored the patient, and encouraged implementation of the evolving care plan. Duties included booking investigations, arranging case conferences, referrals, home visits and collecting data for evaluation of the intervention.
Quality of life was measured by the SF-3611 and the Dartmouth COOP function charts (COOP).12,13 Using the SF-36 as an outcome measure was a requirement of the coordinated care trials, and the physical and mental component summary scores were selected pre-hoc as those most relevant to our study.
Functionality was measured by part of the COOP function charts, the Older Americans Resources and Services independent activities of daily living questionnaire (OARS),14 and the Modified Barthel Index (MBI).15 The COOP was chosen as a simple, graphic questionnaire suitable for frail, elderly people with respiratory disease, including those with limited English.
All questionnaires were administered at baseline and on study termination in December 1999, after at least 12 months' study participation. The SF-36 was administered by telephone. Where telephone contact could not be made because of lack of telephone or insufficient English, the interview was conducted by the service coordinator with the assistance of a family member where necessary. All the other questionnaires were administered by service coordinators. Interviewers were not blinded to the study hypotheses.
Information about hospital admissions for the study period and the previous 12 months was collected for all patients from South Australian Department of Human Services hospital separation data to enable an intention-to-treat analysis. Patients can be identified across hospitals and admissions in more than 90% of cases through Medicare numbers. Unplanned admissions were coded as a separate field in this data source, and respiratory admissions were identified by Australian National Diagnosis Related Groups16 176 (pulmonary oedoma and respiratory failure) or 177 (chronic obstructive airways disease).
Medical Benefits Scheme (MBS), Pharmaceutical Benefits Scheme (PBS) and Department of Veterans' Affairs (DVA) data were obtained from the Health Insurance Commission (HIC).17 Each participant received an explanation of the rationale for collecting these data, and, if agreeable, filled out a consent form for data release. Information regarding deaths was obtained from the SA Death Register through the Department of Human Services.
Cox proportional hazards regressions (adjusted for age and previous hospitalisation) were used to check for differences in the length of time in the trial between groups, and to check for differences in time until death between groups.
For the baseline demographic and prior health service utilisation data and the baseline quality-of-life characteristics, unadjusted comparisons between groups were performed using χ2 test, t test or Wilcoxon rank-sum test, as applicable. Changes in SF-36 component summary scores were compared between groups using multiple linear regression on a subset of participants who completed both SF-36 questionnaires, adjusting for five potential confounders (MBS expenditure and hospitalisation in the 12 months before the study, age, sex, and smoking).
Improvements in functionality were assessed by counting the number of people in each group considered better, the same or worse than baseline at the end of the study, with adjustment for potential confounders (the same five as for the SF-36 analysis) using a multiple ordered logit model.15
For both the SF-36 and improvements-in-functionality analysis, only people who completed baseline and follow-up questionnaires were included. Consequently, adjustment for length of time in the study was not required.
Differences in hospitalisation for any reason, respiratory admission and unplanned admission were examined using multiple logistic regression, adjusting for the same five confounders. Analysis was on an intention-to-treat basis. Length of time in the study was not a significant predictor and so was omitted. Average length of stay was analysed using a t test, including only those patients who had been hospitalised.
All analyses were completed using STATA statistical software.18
The national evaluators of the coordinated care trials performed power calculations on a trial-wide basis based on potential changes to SF-36 scores. For our study, power calculations19 were performed for both quality of life and hospitalisation. For a change of 10 points on the 100-point scale of the SF-36 component summary scores (SD, 10; power, 90%; α = 0.05, using a two-sided t test and an intervention : control ratio of 2:1) a sample size of 51 (34 intervention; 17 control) would be required. For a 50% reduction in the incidence of hospitalisation over a 12-month period from a baseline of 42% (power, 90%; α = 0.05, using a χ2 test for an intervention : control ratio of 2:1) the sample size required would be 245 (163 intervention; 82 control).
The Western Respiratory Project involved 223 intervention patients, 154 comparison patients, 92 care coordinator GPs, and six service coordinators (Box 1). The difference in follow-up time was not significant (hazard ratio, 0.89; 95% CI, 0.64–1.25). Thirty patients had less than 90 days' follow-up (intervention, 25; comparison, 5) due to an adjustment being made to the study start date after recruitment (these patients were not included in the analysis). The intervention period varied between patients because of an extended recruitment period with an associated shortening of the period available for the intervention. One hundred and eighty-two subjects did not complete the study (Box 1). The difference in death rates between the groups was not significant (hazard ratio, 0.62; 95% CI, 0.29–1.28) when adjusted for age and previous hospital admissions.
At entry to the study, the median age of the intervention group was 10 years older than the geographic comparison group (P < 0.001). The intervention group had a lower proportion of women (45%, compared with 62% in the comparison group), were less likely to smoke (P = 0.014), less likely to speak English at home (P < 0.001), and had higher rates of hospitalisation in the previous 12 months (P < 0.001). In addition, the intervention group had worse SF-36 physical component summary scores (P < 0.001).
About 90% of participants completed each quality-of-life questionnaire at the beginning of the study (Box 1). The lowest response rate of 71% was attained in the intervention group for the SF-36. These non-respondents were more likely to withdraw from the study, less likely to have had eight GP visits in the previous 12 months, and more likely to have been born outside Australia and not to speak English at home. Similar characteristics were demonstrated by non-respondents for each of the questionnaires.
The baseline COOP function charts indicated that the intervention group were more likely to report diminished functioning (physical condition, breathlessness) and a poorer perception of their overall health and quality of life. Half the intervention group required assistance in at least one task of daily living measured by OARS. There was no overall difference between intervention and comparison groups in activities of daily living measured by the MBI.
Changes in quality of life and functionality scores are summarised in Box 2. Multivariate analysis showed no significant difference in change in SF-36 physical component score. The overall mental component score improved with coordinated care.
The intervention group experienced less deterioration in two out of three symptom-related COOP items (emotional condition and pain) and an improvement in COOP perceived quality of life. There was no difference between the groups in respect to functional COOP or OARS items.
On average, a person receiving coordinated care in the Western Respiratory Study incurred $8312 per year, including an initial $40 enrolment cost, compared with an average of $6882 per year for a person receiving usual care (Box 3). Modifying cost outliers to two standard deviations from the mean did not lead to any significant change in the results for healthcare costs compared with including outliers at full cost. This demonstrates that outliers had little impact.
We studied the effects of coordinated care in people with chronic and complex respiratory disease, and found a reduced deterioration in mental aspects of quality of life, symptoms of pain and emotional condition, but no difference in physical aspects or functional measures. We found no cost saving to the healthcare system and no reduction in hospital admissions.
Key limitations of the study include the lack of a study sampling frame. Non-participants are not recorded and caution must be taken in generalising the results. Further, our geographical comparison group was not as similar to the intervention group as intended, requiring many adjustments in the analyses for confounders such as age and previous hospitalisation. The higher prevalence of older men, who were less likely to speak English at home, suggested that some GPs in the intervention region had patients of a considerably different demographic background to subjects recruited by GPs in the comparison region. This study design, and the extent of associated adjustments, may have weakened the validity of the differences in outcomes.
The action research approach and lack of interviewer blinding may have limited scientific rigour. Although this project was a compromise between scientific evaluation and action research, it provided an important, large scale, community-based and shared healthcare provider intervention. Out of necessity, the design was flexible to adapt to the requests of multiple stakeholders, particularly GPs and their patients, for the duration of the study. Without such flexibility, implementation across two broad geographical regions would have been impractical, and it is doubtful that GPs would have agreed to initiate or maintain involvement in the study.
Although the study had sufficient power to show differences in admissions and quality of life, we found no reduction in admissions, and only modest positive benefit with respect to quality of life. This might be primarily due to further limitations of the study. Firstly, factors such as the variation in the period of the intervention and the high dropout rate reduced the potential for demonstrating an effect. Such factors are not unexpected in studies of elderly, chronically ill subjects in community settings. Secondly, owing to patient de-identification in the study database, we were unable to link patients and GPs in the comparison group. This meant that we were unable to adjust for clustering by GP in the analysis. However, as most GPs had only 1–3 patients (intervention 223 patients, 92 GPs; comparison 154 patients, 70 GPs), the clustering effects are expected to be minor.
The increased dropout rate immediately after 12 months in the intervention group appears to have resulted from dissatisfaction with the interview questionnaire. This is an important consideration when lengthy quality-of-life and other measures are being asked of often frail, elderly, chronically ill participants.21
Our results suggest that a general practice-based intervention such as coordinated care may be the wrong approach for people with chronic respiratory disease. Many patients with advanced respiratory disease may be so ill that coordinated care can have little effect on the course of the illness, the patient's well being, or healthcare service utilisation. It is difficult for large community studies such as the coordinated care trials to target specific subgroups of the population with chronic respiratory disease owing to the large participant numbers that such studies require. It may be better in future to focus on locally targeted programs, such as pulmonary rehabilitation. Pulmonary rehabilitation has demonstrated benefits in chronic obstructive pulmonary disease,22 and a home-based program could be incorporated with home-based management to improve future coordinated care interventions for respiratory disease.
A Cochrane review of a large, well-designed outreach nursing care study for patients with chronic obstructive pulmonary disease2 also showed only modest improvements in quality of life, accompanied by a substantial increase in healthcare system costs.23 Congruent findings have been demonstrated in a locally conducted outreach respiratory nurse program, and by a systematic review of 15 studies of preventive home care visits in elderly patients.24 The value of modest increases in quality of life in people with complex and chronic conditions, for substantial increase in healthcare costs, needs to be considered at a societal level. Given the many difficulties reported with coordinated care in this study and the lack of cost benefits, the future of wide-ranging coordinated care interventions looks limited. Since the introduction in 2001 of the Enhanced Primary Care items to the MBS (for health assessments, care plans and case conferences), it is unclear whether the revised costs of coordinated care will still outweigh any demonstrated benefits, but the problems encountered in this study are likely to hinder future attempts to show an overall benefit.
2: Percentage of patients who showed improvement or deterioration in functionality and quality of life*
Control (C) (usual care) |
Intervention (I) (coordinated care) |
P † |
|||||||||
Deteriorated |
Same |
Improved |
Deteriorated |
Same |
Improved |
||||||
SF-36 physical (n = 150; C, 67; I, 83) |
15% |
73% |
12% |
13% |
72% |
15% |
0.627 |
||||
SF-36 mental (n = 150; C, 67; I, 83) |
24% |
66% |
10% |
16% |
59% |
25% |
0.023 |
||||
MBI (n = 147; C, 59; I, 88) |
7% |
80% |
13% |
23% |
68% |
9% |
0.080 |
||||
OARS (n = 150; C, 61; I, 89) |
25% |
52% |
23% |
28% |
42% |
30% |
0.226 |
||||
COOP |
|||||||||||
Physical condition (n = 159; C, 65; I, 94) |
29% |
48% |
23% |
21% |
63% |
16% |
0.528 |
||||
Daily activities (n = 160; C, 65; I, 95) |
28% |
46% |
26% |
31% |
46% |
23% |
0.785 |
||||
Social activities (n = 159; C, 65; I, 94) |
37% |
43% |
20% |
32% |
46% |
22% |
0.162 |
||||
Emotional (n = 160; C, 65; I, 95) |
46% |
31% |
23% |
24% |
51% |
25% |
0.008 |
||||
Breathlessness (n = 158; C, 65; I, 93) |
29% |
52% |
19% |
25% |
54% |
22% |
0.162 |
||||
Pain (n = 160; C, 65; I, 95) |
43% |
29% |
28% |
21% |
54% |
25% |
0.031 |
||||
Overall condition (n = 160; C, 65; I, 95) |
37% |
35% |
28% |
19% |
51% |
31% |
0.072 |
||||
Quality of life (n = 160; C, 65; I, 95) |
31% |
49% |
20% |
21% |
49% |
29% |
0.030 |
||||
Social support (n = 160; C, 65; I, 95) |
22% |
60% |
18% |
11% |
68% |
21% |
0.076 |
||||
* Improvement or deterioration means a change in SF-36 score of 12 or more, and a change of one unit on the five-item COOP and MBI scales and on the 15-item OARS scale. † Except for the SF-36 comparisons, P values are adjusted for MBS expenditure in the previous 12 months, age, sex, smoking and hospitalisation in the previous 12 months. |
|||||||||||
3: Recalibrated costs per patient-year for coordinated care and usual care
Usual care |
Coordinated care |
Difference |
Recalibration factor |
||||||||
MBS |
$980 |
$1111 |
$131 |
1.003 |
|||||||
PBS |
$909 |
$1038 |
$129 |
1.254 |
|||||||
Hospital inpatient |
$4856 |
$5035 |
$179 |
2.079 |
|||||||
Other |
$137 |
$257 |
$120 |
1.355 |
|||||||
Enrolment |
$0 |
$40 |
$40 |
||||||||
Care planning |
$0 |
$182 |
$182 |
||||||||
Coordination |
$0 |
$649 |
$649 |
||||||||
Total |
$6882 |
$8312 |
$1430 |
||||||||
Data not available for Department of Veterans' Affairs and hospital (non-inpatient) costs. MBS = Medical Benefits Scheme. PBS = Pharmaceutical Benefits Scheme. |
|||||||||||
Received 7 November 2001, accepted 5 June 2002
- Brian J Smith1
- Heather J McElroy2
- Richard E Ruffin3
- Adrian R Heard4
- Peter A Frith5
- Malcolm W Battersby6
- Adrian J Esterman7
- Peter Del Fante8
- Peter J McDonald9
- 1 The Queen Elizabeth Hospital, Adelaide, SA.
- 2 Repatriation General Hospital, Daw Park, SA.
- 3 Flinders Medical Centre, Adelaide, SA.
- 4 Adelaide Western Division of General Practice, Adelaide, SA.
- 5 SA HealthPlus, Adelaide, SA.
We thank Peter Michaels, Phillip Widdas, Dawn Kroemer, Elizabeth Kalucy, Chris McGowan and Jesia Berry for their considerable contribution to the study. We also recognise the input of the various participants, service coordinators, care coordinators, and HealthPlus team members. This study was primarily funded by the Commonwealth Department of Health and Aged Care, Coordinated Care Trials, Canberra, in conjunction with the Department of Human Services, SA. We wish to thank the staff of the Acute Care Section, Department of Health and Aged Care, the Information Management Division of the Health Insurance Commission and Health Systems Development Services of the SA Department of Human Services.
None identified.
- 1. Commonwealth Department of Health and Aged Care. The Australian coordinated care trials: background and trial descriptions. Canberra: Department of Health and Aged Care, 1999.
- 2. Smith B, Appleton S, Adams R, et al. Home care by outreach nursing for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2001; 3: CD000994.
- 3. Frith P, Battersby M, McDonald P, McGowan C. Improving processes and clinical care in the SA HealthPlus coordinated care trial. The Australian coordinated care trials — reflections and lessons. Canberra: Publications Production Unit, Commonwealth Department of Health and Aged Care, 2001.
- 4. Glover J. A social health atlas of Australia. Volume 5: South Australia. Adelaide: Openbook Publishers, 1999.
- 5. Batehup L, editor. Facilitating change in nursing practice: studies in action research. London: Churchill Livingstone, 1999.
- 6. Angwin J. The essence of action research. Geelong: Deakin Centre for Education and Change, Deakin University, 1998.
- 7. Battersby M, McDonald P, Pearce R, et al. The changing attitudes of health professionals and consumers towards a coordinated care trial – SA HealthPlus. Aust Health Rev 2001; 24: 172-178.
- 8. Thoracic Society of Australia & New Zealand. Guidelines for the management of chronic obstructive pulmonary disease. Mod Med Aust 1995; 38: 132-146.
- 9. British Thoracic Society. BTS guidelines for the management of chronic obstructive pulmonary disease. The COPD Guidelines Group of the Standards of Care Committee of the BTS. Thorax 1997; 52 Suppl 5: S1-S28.
- 10. American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995; 152: S77-S121.
- 11. Australian Bureau of Statistics. National Health Survey Australia, 1995: SF-36 population norms. Canberra: ABS, 1997. (Catalogue no. 4399.0.)
- 12. Landgraf JM, Nelson EC, Dartmouth COOP Primary Care Network. Summary of the WONCA/COOP International Health Assessment Field Trial. Aust Fam Physician 1992; 21: 255-269.
- 13. Bowling A. Measuring health: a review of quality of life measurement scales. Buckingham: Open University Press, 1997.
- 14. Fillenbaum GG. Screening the elderly: a brief instrumental activities of daily living measure. J Am Geriatr Soc 1985; 33: 698-705.
- 15. Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel index for stroke rehabilitation. J Clin Epidemiol 1989; 42: 703-709.
- 16. Commonwealth Department of Health and Family Services and 3M Health Information Systems. Australian National Diagnosis Related Groups definitions manual version 3.1. Wallingford: 3M Health Information Systems, 1996.
- 17. Health Insurance Commission. Understanding HIC data. 7 July 2000. <http://www.hic.gov.au/hinfo/hicdata.pdf>. Accessed 16 October 2000, no longer available.
- 18. STATA statistical software [computer program]. Version Release 6.0. College Station, TX: Stata Corporation, 1999.
- 19. Lwanga SK, Lemeshow S. Ssize [computer program]. Version 2. Geneva: WHO, 1998.
- 20. Keeley S, Reid S. Short form (36) SA health status assessment study — ambulatory care. Adelaide: SA Health Commission, 1997.
- 21. Smith BJ, Appleton SL, Bennett PW, et al. The effect of a respiratory home nursing intervention in patients with chronic obstructive pulmonary disease (COPD). Aust N Z J Med 1999; 29: 718-725.
- 22. Benzo R, Flume PA, Turner D, Tempest M. Effect of pulmonary rehabilitation on quality of life in patients with COPD: the use of SF-36 summary scores as outcome measures. J Cardiopulm Rehabil 2000; 20: 231-234.
- 23. Bergner M, Hudson L, Conrad D, et al. The cost and efficacy of home care for patients with chronic lung disease. Med Care 1988; 26: 566-579.
- 24. van Haastregt JCM, Diederiks JPM, van Rossum E, et al. Effects of preventive home visits to elderly people living in the community: systematic review. BMJ 2000; 320: 754-758.





Abstract
Objectives: To evaluate the effectiveness of coordinated care for chronic respiratory disease.
Design and setting: Community-based geographical control study, in western (intervention) and northern (comparison) metropolitan Adelaide (SA).
Participants: 377 adults (223 intervention; 154 comparison) with chronic obstructive pulmonary disease, asthma or other chronic respiratory condition, July 1997 to December 1999.
Intervention: Coordinated care (includes care coordinator, care guidelines, service coordinator and care mentor).
Main outcome measures: Hospital admissions (any, unplanned and respiratory), functionality (activities of daily living) and quality of life (SF-36 and Dartmouth COOP).Results: At entry to the study, intervention and comparison subjects were dissimilar. The intervention group was 10 years older (P < 0.001), less likely to smoke (P = 0.014), had higher rates of hospitalisation in the previous 12 months (P < 0.001) and had worse self-reported quality of life (SF-36 physical component summary score [P < 0.001] and four of nine COOP domains [P = 0.002–0.013]). After adjustment for relevant baseline characteristics, coordinated care was not associated with any difference in hospitalisation, but was associated with some improvements in quality of life (SF-36 mental component summary score [P = 0.023] and three of nine COOP domains [P = 0.008–0.031]) compared with the comparison group.
Conclusions: Coordinated care given to patients with chronic respiratory disease did not affect hospitalisation, but it was associated with an improvement in some quality-of-life measures.