Gastro-oesophageal reflux disease (GORD) is frequent in the Australian community, and a very common reason for general practice visits. Data from the United States and the United Kingdom suggest that heartburn and acid regurgitation may occur weekly in up to 20% of the population and monthly in up to 40%.1-3 Similarly high rates have been observed in Australia and New Zealand.4,5
The value of lifestyle measures and antacids in managing GORD remains poorly documented, and their effectiveness is probably limited.6 There has been considerable debate as to whether treatment of patients with GORD should be initiated with H2-receptor antagonists and "stepped up" to more effective agents if treatment fails, or if it should commence with proton-pump inhibitors, currently the most effective therapy, and then be "stepped down" when symptom control is achieved.7,8
GORD is usually relapsing or chronic, and most patients require long-term medical management. Half-dose proton-pump inhibitor treatment represents a potentially attractive long-term option as it is less expensive than full-dose treatment.6 However, there are no Australian data on the long-term effects of low-dose proton-pump inhibitors compared with those of full-dose H2-receptor antagonists in patients with symptomatic GORD. Indeed, few international studies have compared these approaches in primary care.6,7,9
We therefore conducted a randomised, multicentre study to test the hypothesis that low-dose pantoprazole (20 mg once daily) would produce superior symptomatic remission rates than standard-dose ranitidine (150 mg twice daily) in patients presenting to general practitioners with symptoms of GORD.
Ethical approval for the trial protocol was obtained from the Royal Australian College of General Practitioners. All members of the Hornsby Ku-ring-gai Division of General Practice (HKDGP) and the Newcastle and Maitland subregions of the Hunter Urban Division of General Practice (HUDGP) were invited to participate in the study.
We recruited adults aged 18 years and older who presented with symptomatic GORD between 19 January 1999 and 22 September 2000. All participating patients gave written informed consent.
Patients were eligible for the study if they reported experiencing heartburn (defined as "a burning feeling rising from the stomach or lower chest towards the neck"6,10) at least twice a week as the predominant upper-gastrointestinal complaint. Antacid medication and concomitant medications for intercurrent or chronic diseases were permitted during the study. Exclusion criteria are listed in Box 1.
Patients were allocated to treatment groups on the basis of a computer-generated randomisation list administered by participating Divisions of General Practice. The allocation sequence was blinded. No stratification methods were used.
A "double-dummy" design was used to ensure double-blind status of doctor and patients. Patients took either 20 mg of pantoprazole once daily and dummy "ranitidine" (placebo) twice daily, or dummy "pantoprazole" (placebo) once daily and 150 mg of ranitidine twice daily.
Treatment continued for 12 months, symptoms and adverse events were assessed at Weeks 4 and 8, and Months 3, 6, 9 and 12. Patients were asked to assess the severity of their heartburn prospectively by submitting a heartburn diary card recording the level of discomfort due to heartburn, and antacid use, for seven consecutive days at the end of each month, and by completing the Gastrointestinal Symptom Rating Scale (GSRS)10 on the final diary day each month.
Patients were asked to return unused medication for assessment of compliance by tablet counts. Compliance was defined as the consumption of 80%–120% of the expected number of tablets.
The primary endpoint was symptom control rate, measured by comparing heartburn frequency at baseline, six months and 12 months. "Complete symptom control" was defined as the absence of any episodes of heartburn during the seven days before follow-up. "Sufficient symptom control" was defined as a mild episode of heartburn experienced on not more than one day during the seven days before follow-up. The heartburn diary provided a seven-point graded scale ranging from "no discomfort at all" to "very severe discomfort" in response to the question "Please mark the choice that best shows how much heartburn has bothered you for each day".
Secondary endpoints included improvement of GORD symptoms, assessed by monthly GSRS scores, and comparative symptomatic relapse rates, based on heartburn diary records of heartburn frequency. "Symptomatic relapse" was defined as heartburn of at least mild intensity on three of the seven days before follow-up in a patient in whom remission (defined as complete symptom control) had been achieved by Week 8.
The GSRS is a 15-item questionnaire which uses a seven-point graded Likert scale, where 1 represents "no discomfort" and 7 represents "very severe discomfort". Its 15 questions are aggregated into five syndromes — abdominal pain, reflux, indigestion, diarrhoea, and constipation. A syndrome score was calculated for each patient by taking the mean score for all questions relating to the syndrome for each time point.11
A total of 76 general practitioners recruited 307 patients who entered the random-allocation phase; 154 were assigned to the pantoprazole arm and 153 to the ranitidine arm (Box 2). A total of 184 patients completed the study: 101 in the pantoprazole treatment group and 83 in the ranitidine group.
Significantly more patients withdrew from the ranitidine group than the pantoprazole group (70 v 53; P = 0.04), with lack of efficacy being the most common reason. Nineteen (12%) of the pantoprazole group and 22 (14%) of the ranitidine group withdrew because of adverse events, thought to be related to the study medication in 11 patients in each group.
Baseline demographic and clinical characteristics were well matched between the two groups (Box 3). Eighty-eight per cent of the pantoprazole and 85% of the ranitidine group reported moderate to very severe heartburn on entry into the study, with 46% and 53%, respectively, having experienced daily heartburn. Ninety per cent of participants were compliant with the study treatment.
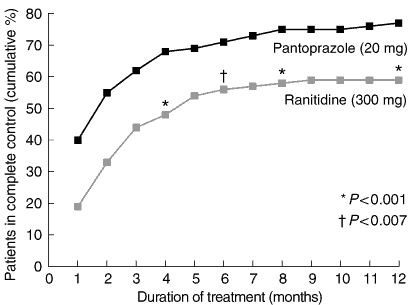
Complete symptom control: By intention-to-treat analysis at Weeks 4 and 8, the rate of complete symptom control was significantly higher in patients treated with pantoprazole than those receiving ranitidine (P < 0.001; Box 4). Most patients who achieved complete symptom control during the study had done so within the first six months of treatment. At Month 6, complete symptom control was reported by 71% of patients receiving pantoprazole and 56% of patients receiving ranitidine (P = 0.007). By the end of the 12-month study period, 77% of the pantoprazole group and 59% of the ranitidine group reported complete symptom control (P = 0.001; Box 4). These results were very similar to those obtained in the per-protocol analysis.
Sufficient symptom control: After four weeks' treatment, 64% of pantoprazole-treated patients, compared with 48% of ranitidine-treated patients, achieved sufficient symptom control (P = 0.008). By 12 months, 86% of the pantoprazole group and 79% of the ranitidine group reported sufficient symptom control; this difference was not significant. Only one patient achieved sufficient symptom control after the six-month point of the study.
Gastrointestinal symptom rating scale scores: Analysis of GSRS scores for each visit showed significantly lower scores for abdominal pain (P = 0.007), reflux (P < 0.001), indigestion (P < 0.001) and diarrhoea (P = 0.04) among patients in the pantoprazole group compared with those in the ranitidine group. However, the absolute differences were small.
Our trial has shown that low-dose pantoprazole once daily was superior to standard-dose ranitidine twice daily for the initial and long-term management of patients with symptomatic GORD in primary care. Patients treated with pantoprazole showed significantly higher rates of complete symptom control after four weeks' treatment, and this remained significant at six and 12 months. Both drugs were well tolerated. The trial was methodologically rigorous, with careful attention paid to patient selection and blinding.
Patients with endoscopy-negative GORD represent the largest subgroup presenting to primary care with chronic reflux symptoms. Of the remainder, most patients have only mild grades of reflux oesophagitis.6 Both low-dose proton-pump inhibitors and full-dose H2-receptor antagonists are usually inadequate for symptom control in severe grades of oesophagitis, but this factor probably accounted for only a few failures in either treatment arm.6 It is more likely that non-responders were mainly patients with endoscopy-negative disease, who probably represent a heterogeneous group.
Few data are available comparing maintenance proton-pump inhibitor therapy with standard H2-receptor antagonist therapy in primary care. In a trial conducted in patients with endoscopy-negative reflux disease, 60% of those receiving omeprazole (10 mg) achieved symptomatic remission at 24 weeks, compared with 24% of those receiving cimetidine.12 Our trial provides direct evidence that, in patients with uninvestigated reflux, low-dose proton-pump inhibitor therapy was superior to full-dose ranitidine, and provided symptom control in 40% of patients within one month and 71% at six months, with a therapeutic gain of 15% at six months. Eighty-six per cent of patients receiving pantoprazole had achieved sufficient symptom control at six months. Pantoprazole was clearly superior to ranitidine in the first month of therapy, although many had not responded by then to either therapy.
Our trial did not examine whether all patients require maintenance treatment. However, another study has assessed intermittent treatment over one year in patients with endoscopy-proven symptomatic reflux disease in a randomised controlled trial.13 Patients initially received standard-dose omeprazole, low-dose omeprazole, or standard-dose ranitidine. Those who became asymptomatic or mildly symptomatic were then followed for 12 months and any recurrences of heartburn were treated with the dose that was successful in initially controlling symptoms. In all treatment groups, about half of the patients did not need any treatment for at least six months. Such data support a management approach based on self-directed "on-demand" therapy as an alternative to continuous therapy, as patients will often apply this approach by taking medication only when troubled by symptoms.6 Randomised placebo-controlled trials have shown that on-demand therapy is effective and well tolerated in patients with endoscopy-negative GORD.13,14
Safety is a key issue for first-line proton-pump inhibitor therapy for symptomatic reflux disease in general practice. We identified no serious adverse events related to misdiagnosis or treatment in our study after one year of follow-up. The clinical relevance of eradicating Helicobacter pylori in those infected before initiating suppression remains controversial, so we did not determine H. pylori status in our present study. Longer-term treatment with a proton-pump inhibitor has been associated with a small, though definite, increase in the incidence of gastric corpus mucosal atrophy in patients infected with H. pylori. In a study of patients with refractory reflux oesophagitis prescribed omeprazole at standard or higher doses, annual incidences of gastric corpus mucosal atrophy among patients positive and negative for H. pylori were 4.7% and 0.7%, respectively.15 Notably, this increase occurred mainly in those who were elderly and had moderate or severe gastritis at baseline. Other investigators have shown that H. pylori eradication prevents this increase in corpus gastritis, although it is unclear whether it also prevents the progression to gastric atrophy.16 An increased incidence of benign fundic gland polyps has been observed in patients taking proton-pump inhibitors, but does not appear to be clinically significant.17 Although rebound acid hypersecretion occurs after ceasing proton-pump inhibitor therapy, it appears to be restricted to patients negative for H. pylori and is related to the degree of pH elevation during treatment.18,19 Moreover, the clinical relevance of this finding is unclear, and is likely to be small.
Potential failure to identify serious underlying disease is another issue for empirical treatment in patients who have not undergone endoscopy. There is some concern that lesions may be missed or misdiagnosed in patients who are subsequently investigated while receiving proton-pump inhibitor therapy. Case studies have documented failure to recognise early gastric cancers during endoscopy in patients prescribed proton pump inhibitors.20,21 However, curable cancer is rarely identified, and endoscopy in patients with GORD is generally considered to contribute only minimally to its diagnosis. For example, a Canadian study of 742 patients who underwent endoscopy for reflux symptoms found that, regardless of the grade of oesophagitis detected, the most frequent resultant management decisions were dose maintenance or increase in those already receiving a proton-pump inhibitor, and switching to proton-pump inhibitor therapy in those receiving an H2-receptor antagonist. Furthermore, no oesophageal cancers were identified and the prevalence of Barrett's oesophagus was very low.22
Overall, the literature suggests that the risk of serious disease is minimal in patients with reflux attending primary care. Furthermore, there is no evidence that oesophagitis grade worsens over a 10-year period, regardless of treatment.6 While patients who have or develop symptoms such as weight loss, vomiting or bleeding require prompt evaluation, preferably before treatment is started, the current data suggest an empirical trial of therapy is generally safe and acceptable in the absence of such symptoms.
Pantoprazole (20 mg daily) has been demonstrated elsewhere to provide adequate long-term maintenance therapy for patients with GORD in whom remission had already been achieved with standard-dose proton-pump inhibitor therapy.23 It has also been demonstrated to be effective and well tolerated in the treatment of patients with ulcerative oesophagitis.24 In our study, low dose pantoprazole was shown to be effective treatment in patients with symptomatic gastro-oesophageal reflux disease in the general practice setting.
In conclusion, low-dose pantoprazole appears to be an effective alternative to a standard dose of ranitidine in the long-term management of symptomatic GORD. An empirical treatment strategy also appears to be safe in general practice, assuming patients with alarm symptoms are excluded.
1: Exclusion criteria
History of proven peptic ulcer
History of Zollinger–Ellison syndrome
Previous surgery of the oesophagus or gastrointestinal tract (with the exceptions of appendicectomy or gallbladder surgery)
History of cirrhosis or severe hepatic disease
Concomitant severe diseases or laboratory abnormalities that, in the opinion of the general practitioner, precluded study enrolment
Use of a proton pump inhibitor, sucralfate, H2-receptor antagonist or prokinetic drug within the previous week
Current medication with any drug with pH-dependent absorption
Long-term use of glucocorticoids or non-steroidal antirheumatic agents
Use of any drug that, in the opinion of the GP, precluded study enrolment
Allergy to proton-pump inhibitors or H2-receptor antagonists
History of alcohol or drug abuse
Participation in a clinical study within the past two months
Concurrent use of other investigational medications
Inability to comply with the study protocol
Pregnancy and lactation
Childbearing potential in the absence of intention to use adequate contraceptive measures for the duration of the study
2: Establishing the study population for a randomised controlled trial of pantoprazole (20 mg once daily) versus ranitidine (150 mg twice daily) for the treatment of heartburn
a) Recruitment and random allocation
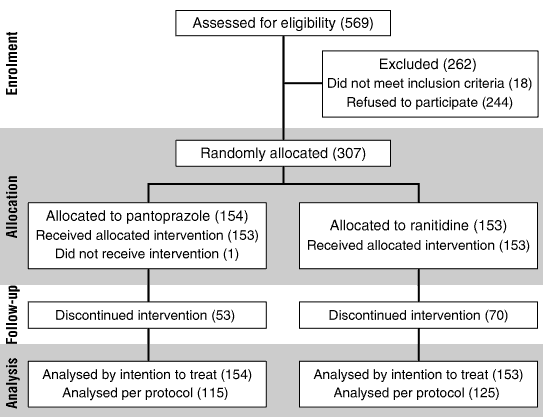
b) Reasons for patient withdrawal*
Pantoprazole |
Ranitidine |
||||||||||
Total patients |
154 |
153 |
|||||||||
Number withdrawn† |
53 (34%) |
70 (46%) |
|||||||||
Consent withdrawn |
19 (12%) |
12 (8%) |
|||||||||
Poor efficacy |
16 (10%) |
36 (24%) |
|||||||||
Adverse event(s) related to study medication |
11 (7%) |
11 (7%) |
|||||||||
Adverse event(s) not related to study medication |
8 (5%) |
11 (7%) |
|||||||||
Protocol deviation |
3 (2%) |
8 (5%) |
|||||||||
Lost to follow-up |
1 (1%) |
3 (2%) |
|||||||||
Other |
5 (3%) |
8 (5%) |
|||||||||
* Difference between withdrawal rates of pantoprazole and ranitidine treatments significant at P = 0.04. † Patients are counted only once in the "Number withdrawn" row, but may have withdrawn for multiple reasons, and are included in the count for each applicable reason for withdrawal. |
|||||||||||
3: Baseline characteristics of study participants
Pantoprazole (n = 154) |
Ranitidine (n = 153) |
||||||||||
Demographics |
|||||||||||
Female |
78 (51%) |
83 (54%) |
|||||||||
White |
150 (97%) |
146 (95%) |
|||||||||
Non-smoker |
89 (58%) |
92 (60%) |
|||||||||
Mean age (years)* |
53 (19–87) |
52 (19–80) |
|||||||||
Mean weight (kg)* |
82 (48–252) |
80 (48–148) |
|||||||||
Mean height (m)* |
1.68 (1.4–1.96) |
1.68 (1.36–1.90) |
|||||||||
Mean BMI (kg/m2)* |
28.9 (17.9–72.7) |
28.4 (17.6–51.9) |
|||||||||
Mean GSRS score* |
20 (3–60) |
21 (2–55) |
|||||||||
Alcohol use |
|||||||||||
Occasional |
91 (59%) |
83 (54%) |
|||||||||
Daily |
28 (18%) |
30 (20%) |
|||||||||
Heartburn frequency |
|||||||||||
Several times per week |
83 (54%) |
72 (47%) |
|||||||||
Daily |
71 (46%) |
81 (53%) |
|||||||||
Heartburn severity |
|||||||||||
Slight discomfort |
1 (1%) |
1 (1%) |
|||||||||
Mild discomfort |
17 (11%) |
23 (15%) |
|||||||||
Moderate discomfort |
65 (42%) |
50 (33%) |
|||||||||
Moderately severe discomfort |
39 (25%) |
53 (35%) |
|||||||||
Severe discomfort |
28 (18%) |
23 (15%) |
|||||||||
Very severe discomfort |
4 (3%) |
3 (2%) |
|||||||||
Duration of heartburn symptoms |
|||||||||||
Less than 1 year |
17 (11%) |
22 (14%) |
|||||||||
1–5 years |
50 (32%) |
42 (27%) |
|||||||||
6–10 years |
32 (21%) |
39 (26%) |
|||||||||
More than 10 years |
55 (36%) |
50 (33%) |
|||||||||
Response to antacids |
|||||||||||
Heartburn relieved by antacids |
104 (68%) |
117 (76%) |
|||||||||
Heartburn not relieved by antacids |
22 (14%) |
19 (12%) |
|||||||||
Antacids not taken |
28 (18%) |
17 (11%) |
|||||||||
* Mean score (range). BMI = Body Mass Index. GSRS = Gastrointestinal Symptom Rating Scale. |
|||||||||||
Received 7 November 2001, accepted 27 June 2002
- Nicholas J Talley
- Michael G Moore2
- Arn Sprogis3
- Peter Katelaris4
- 1 University of Sydney, Nepean Hospital, Penrith, NSW.
- 2 Hornsby Ku-ring-gai Ryde Division of General Practice, Wahroonga, NSW.
- 3 Hunter Urban Division of General Practice, Newcastle, NSW.
- 4 Department of Gastroenterology, University of Sydney, Concord Hospital, Concord, NSW.
We thank Kerry Allanson, Helen Hendrie, Michelle Hancock of the Hunter Urban Division of General Practice, and Lyndall Henderson, Liesel Whyte, Christine Aiken, Pam Webster of Hornsby Ku-ring-gai Ryde Division of General Practice, for the operational management of the study. The study drug, pantoprazole, was supplied by Byk Gulden Pty Ltd, Germany.
Professor Talley has been a consultant to Pharmacia, Janssen-Cilag, Novartis and Astra-Zeneca, and has received research funding from these companies and from Lederle. Dr Katelaris has been a consultant to Pharmacia, Janssen-Cilag and AstraZeneca. Dr Sprogis and Dr Moore have not received personal financial support from the sponsor. This study was initiated and funded by Pharmacia Australia Pty Limited.
- 1. Howard PJ, Heading RC. Epidemiology of gastroesophageal reflux disease. World J Surg 1992; 16: 288-293.
- 2. Talley NJ, Zinsmeister AR, Schleck CD, Melton LJ. Dyspepsia and dyspepsia subgroups: a population-based study. Gastroenterology 1992; 102: 1259-1268.
- 3. Jones R, Lydeard S. Prevalence of symptoms of dyspepsia in the community. BMJ 1989; 298: 30-32.
- 4. Haque M, Wyeth JW, Stace NH, et al. Prevalence, severity and associated features of gastro-oesophageal reflux and dyspepsia: a population-based study. N Z Med J 2000; 113: 178-181.
- 5. Westbrook JI, McIntosh J, Talley NJ. Factors associated with consulting medical or non-medical practitioners for dyspepsia: an Australian population-based study. Aliment Pharmacol Ther 2000; 14: 1581-1588.
- 6. Dent J, Brun J, Fendrick AM, et al. An evidence-based appraisal of reflux disease management — the Genval Workshop Report. Gut 1999; 44(Suppl2): S1-S16.
- 7. Galmiche J-P. How do we offer clinical relief to patients with gastro-oesophageal reflux disease? Eur J Gastroenterol Hepatol 2000; 12(Suppl 1): S3-S6.
- 8. De Vault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 1999; 94: 1434-1442.
- 9. Dent J, Jones R, Kahrilas PJ, Talley NJ. Management of gastro-oesophageal reflux disease in general practice. BMJ 2001; 322: 344-347.
- 10. Carlsson R, Dent J, Bolling-Sternevald E, et al. The usefulness of a structured questionnaire in the assessment of symptomatic gastroesophageal reflux disease. Scand J Gastroenterol 1998; 33: 1023-1029.
- 11. Revicki DA, Wood M, Wicklund I, et al. Reliability and validity of the gastrointestinal symptom rating scale in patients with gastroesophageal reflux disease. Qual Life Res 1998; 7: 75-83.
- 12. Bate CM, Green JR, Axon AT, et al. Omeprazole is more effective than cimetidine in the prevention of recurrence of GERD-associated heartburn and the occurrence of underlying oesophagitis. Aliment Pharmacol Ther 1998; 12: 41-47.
- 13. Bardhan KD, Muller-Lissner S, Bigard MA, et al. Symptomatic gastro-oesophageal reflux disease: double blind controlled study of intermittent treatment with omeprazole or ranitidine. The European Study Group. BMJ 1999; 318: 502-507.
- 14. Talley NJ, Lauritsen K, Tunturi-Hihnala H, et al. Esomeprazole 20 mg maintains symptom control in endoscopy-negative gastro-oesophageal reflux disease: a controlled trial of "on-demand" therapy for 6 months. Aliment Pharmacol Ther 2001; 15: 347-54.
- 15. Klinkenberg-Knol EC, Nelis F, Dent J, et al. Long-term omeprazole treatment in resistant gastroesophageal reflux disease: efficacy, safety, and influence on gastric mucosa. Gastroenterology 2000; 118: 661-669.
- 16. Schenk BE, Kuipers EJ, Nelis GF, et al. Effect of Helicobacter pylori eradication on chronic gastritis during omeprazole therapy. Gut 2000; 46: 615-621.
- 17. Choudhry U, Boyce HW, Coppola D. Proton pump inhibitor-associated gastric polyps: a retrospective analysis of their frequency, and endoscopic, histologic, and ultrastructural characteristics. Am J Clin Pathol 1998; 110: 615-621.
- 18. Gillen D, Wirz AA, Ardill JE, McColl KE. Rebound hypersecretion after omeprazole and its relation to on-treatment acid suppression and Helicobacter pylori status. Gastroenterology 1999; 116: 239-247.
- 19. Waldum HL, Arnestad JS, Brenna E, et al. Marked increase in gastric acid secretory capacity after omeprazole treatment. Gut 1996; 39: 649-653.
- 20. Bramble MG, Suvakovic Z, Hungin AP. Detection of upper gastrointestinal cancer in patients taking antisecretory therapy before gastroscopy. Gut 2000; 46: 464-467.
- 21. Wayman J, Hayes N, Raimes SA, Griffin SM. Prescription of proton pump inhibitors before endoscopy. A potential cause of missed diagnosis of early gastric cancers. Arch Fam Med 2000; 9: 385-388.
- 22. Blustein PK, Beck PL, Meddings JB, et al. The utility of endoscopy in the management of patients with gastroesophageal reflux symptoms. Am J Gastroenterol 1998; 93: 2508-2512.
- 23. Plein K, Hotz J, Wurzer H, et al. Pantoprazole 20 mg is an effective maintenance therapy for patients with gastro-oesophageal reflux disease. Eur J Gastroenterol Hepatol 2000; 12: 425-432.
- 24. Richter JE, Bochenek W, and the Pantoprazole US GERD Study Group. Oral pantoprazole for ulcerative esophagitis: a placebo-controlled, randomized clinical trial. Am J Gastroenterol 2000; 95: 3071-3080.





Abstract
Objectives: To investigate whether pantoprazole (20 mg/d) produces significantly greater symptom control than ranitidine (300 mg/d) in patients with gastro-oesophageal reflux disease (GORD).
Design: Multicentre, randomised, double-blind, parallel-group comparison.
Setting: 76 general practices in north-west Sydney and Newcastle, New South Wales (Australia), from 19 January 1999 to 22 September 2000.
Patients: 307 patients aged 18 years or over presenting with symptomatic GORD.
Interventions: Pantoprazole (20 mg once daily) or ranitidine (150 mg twice daily).
Main outcome measures: Patient-assessed frequency and severity of heartburn using the Gastrointestinal Symptom Rating Scale (GSRS) and a patient heartburn diary.
Results: Pantoprazole was associated with significantly higher rates of complete control of GORD symptoms than ranitidine at four weeks (40% v 19%; P < 0.001), eight weeks (55% v 33%; P < 0.001), six months (71% v 56%; P = 0.007) and 12 months (77% v 59%; P = 0.001).
Conclusions: Low-dose pantoprazole is an effective alternative to standard-dose ranitidine for initial and maintenance treatment of patients with symptomatic GORD.