Coronary heart disease is the most common cause of death in developed countries. The past two decades have seen improvements in therapy that have led to declines in coronary heart disease in countries such as the United States and Australia, with much of this decline being attributable to improvements in care of patients with established disease.1 These improvements include immediate treatment of myocardial ischaemia, such as thrombolytics and aspirin, and better control of risk factors, such as blood pressure, smoking, and cholesterol. The use of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors has been established as the most important means of reducing the toll from hyper-cholesterolaemia. The Scandinavian Simvastatin Survival Study (4S) showed a 30% reduction in the risk of death at a median follow-up of 5.4 years in a group of patients with established coronary heart disease and elevated cholesterol levels (5.5–8.0 mmol/L).2 More recently, the Cholesterol and Recurrent Events (CARE) trial3 and the Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) trial4 have shown that this advantage also applies to patients with established coronary heart disease and average cholesterol levels (4.0–7.0 mmol/L).
At the same time as these advances in therapy, health expenditure has increased, particularly for pharmaceutical products, and a consequence is concern with controlling rising costs. Those who pay for health services increasingly ask about the benefits received for resources invested, either informally or through processes such as cost-effectiveness analysis.5 The governments of Australia and Ontario (Canada) have taken this furthest by requiring information on economic outcomes to assist decisions on subsidising new pharmaceutical products.
To answer the question whether pravastatin is economically worthwhile for patients with established coronary heart disease and "average" cholesterol levels, we undertook a prospective cost-effectiveness analysis within the LIPID trial of pravastatin. The results of the main study and the protocol for the cost-effectiveness component have been published.4,6 In this article, we report the differences in use of resources and quality of life between the pravastatin and placebo groups, and the cost-effectiveness of pravastatin versus placebo for the population of patients in the LIPID trial.
The LIPID trial was a double-blind, randomised, placebo-controlled trial evaluating the long-term effects of pravastatin on all-cause mortality and coronary disease mortality in patients who had had unstable angina or an acute myocardial infarction, and had a total cholesterol level of 4.0–7.0 mmol/L. The trial involved 9014 patients at 85 centres in Australia and New Zealand.4,6 Patients were given dietary advice conforming with the National Heart Foundation's recommendations and randomly assigned to receive 40 mg of pravastatin or placebo daily. Patients were recruited between June 1990 and December 1992, and follow-up was to be at least five years.
The cost-effectiveness substudy aimed to estimate the cost per death averted, the cost per life-year gained, and the cost per quality-adjusted life-year gained. The data requirements were broken down into four major elements:
Data on survival and hospitalisations were collected as part of the main LIPID study. To obtain the additional information required to ascertain cost-effectiveness, several substudies were established. Only the direct costs of healthcare were included, reflecting a healthcare perspective following the Australian Pharmaceutical Benefits Advisory Committee guidelines.7 Costs are reported in 1998 Australian dollars.
All hospitalisations were included and assigned a cost based on the diagnosis-related group category and length of stay.7 Data on long-term (out-of-hospital) medication use were collected as part of the main study, with information about dosage being collected for a separate subgroup (n = 1100). These two sources were combined to estimate total medication use. Drugs were sorted by absolute difference in a month's use (between pravastatin and placebo) and costed until the sum of the absolute cost difference in the last five drugs contributed less than 1% of a cumulative cost difference. For the 91 drugs identified for costing in this way, average doses and frequency of use per month were estimated from the combined-arm substudy of 1100 patients and applied to reported months used to estimate total use in each arm. Substudies of resource use by treatment arm were undertaken for outpatient visits to doctors and other healthcare professionals in the quality-of-life cohort of 1112 patients; use of diagnostic tests in a sample of 485 patients at one, three and five years' follow-up; and nursing home costs, estimated for all 330 patients who experienced stroke and consequently stayed in nursing homes.
For outpatient visits and diagnostic tests, as substudies were based on a sample rather than the full cohort, the common average resource use per patient was inferred, unless there were statistically significant differences by treatment arm in sampled average use. We adjusted the costs of sampled resource use for survival differences between treatment arms.
In the cost-effectiveness analyses, unit costs of resources were allocated as prescribed by the Australian Pharmaceutical Benefits Scheme's manual of costs used for cost-effectiveness analyses.8 Principally, the sources were diagnosis-related group costs for hospitalisations; the Australian Medicare Benefits Schedule9 for outpatient visits and outpatient diagnostic testing; and the schedule of pharmaceutical benefits10 for the costs of medications. Data on average daily dosage from community samples were compared with the substudy medication dosage information to confirm that LIPID patients were similar to a community sample.
The quality-of-life data were obtained from a subcohort of 1112 patients given questionnaires at baseline, and one, three and five years later. The questionnaire was the utility-based quality-of-life questionnaire (UBQ-H), a modification of the York Health Measurement Questionnaire, which was extended to include questions on cardiovascular symptoms and a self-completed time trade-off question.11,12
The analysis used an intention-to-treat principle for both effects and costs. This required that resource use was counted according to a patient's initial randomisation, whether or not he or she continued study medication. Thus, for patients on placebo who "dropped in" by commencing a cholesterol-lowering agent, all medication costs, including the costs of statins, were attributed to the placebo group. Similarly, pravastatin in the active group was costed on an as-dispensed basis. Analogous to the inclusion of all-cause mortality as an outcome, all hospitalisation costs (cardiovascular and non-cardiovascular) were included.
Ninety-five percent confidence intervals for incremental costs (the difference in costs between a pravastatin and placebo patient) were calculated from individual patient total costs, where available, for pravastatin, other medication and hospitalisations. Other incremental costs were considered constant and included as such in the 95% CIs for total incremental costs. A 95% CI for ratios of incremental cost per life saved were estimated from bootstrapping the incremental cost-effectiveness ratio distribution following the method of Briggs et al,13 using individual patient's cost and effect pairs, by treatment arm, in 10 000 bootstrap replications. A constant incremental cost was included for outpatient visits, diagnostic tests and nursing home substudy costs. The same bootstrap replications were also used to estimate a 95% CI for absolute risk reduction in all-cause mortality.
From June 1990 until December 1992, 9014 patients (5958 from Australia and 3056 from New Zealand) from 85 centres were randomly allocated to pravastatin or placebo. Baseline characteristics were well balanced in the two groups. The qualifying event was acute myocardial infarction in 64%, and unstable angina in 36%, with 12% having had both qualifying events (they were included in the myocardial infarction stratum). Of the patients, 17% were women, and 32% had had coronary revascularisation surgery. Their average cholesterol levels were 5.65 mmol/L (total), 3.88 mmol/L (low-density lipoprotein [LDL]), and 0.92 mmol/L (high-density lipoprotein [HDL]). The mean follow-up period was 6.0 years; during this time, 23.8% of patients on placebo commenced cholesterol-lowering therapy (drop-ins), and 18.9% of patients on pravastatin therapy discontinued (drop-outs), of whom 22% went on to another cholesterol-lowering agent.
The cumulative all-cause mortality in the placebo group was 14.1%, and in the pravastatin group was 11.0%, representing a relative reduction of 23% (P < 0.001). This included a 25% relative reduction in cardiovascular deaths (P < 0.001), a 24% reduction in coronary deaths (P < 0.001), and no difference in death rates for non-cardiovascular causes. The rates of non-haemorrhagic stroke were 4.4% in the placebo group and 3.4% in the pravastatin group, a relative reduction of 23% (P = 0.02).
Hospitalisation rates, average unit costs for each admission type, and costs adjusted for long length-of-stay outliers according to casemix standards for New South Wales 1997–19987 are shown in Box 1.
The costs of pravastatin dispensed to the treatment group over the entire trial period were based on a single 40 mg tablet per day, which currently costs $80.30 (Australian dollars) for 30 days' supply on the Pharmaceutical Benefits Scheme. The equivalent cost in the United Kingdom is £31.80 and, in the United States, is $US96.32 (average US wholesale price reduced by 18.3% to reflect that it overestimates actual pharmacy acquisition costs). The 4512 patients used pravastatin for an average of 61.2 months (of the 73 months of follow-up), resulting in an average cost of $4913 per patient. Some other medication costs differed between the two groups (Box 2). Because of the drop-ins to cholesterol-lowering therapy, the placebo group used more cholesterol-lowering agents (apart from study pravastatin) than the pravastatin group. Furthermore, rates of use of some other cardiovascular agents, such as glyceryl trinitrate, nifedipine and metoprolol, were also higher in the placebo group. The net difference in cost for drugs other than pravastatin was $360, representing a 7% cost offset for the cost of pravastatin.
Average resource use did not differ significantly between the placebo and pravastatin groups for ambulatory care (sample of 1112 patients) or diagnostic tests (sample of 485 patients). When the costs of common resource use were extrapolated to allow for survival difference by treatment arm, the incremental costs for the pravastatin group were $45 per person for outpatient visits and $13 for diagnostic tests over the study period (Box 3).
Although there were fewer nursing-home stays after stroke among patients in the pravastatin group than the placebo group (181 v 149), longer average nursing-home stays in the pravastatin group (49.1 v 37.9 days) resulted in greater costs over the study period, equivalent to $21 per person (Box 4).
Quality of life, as measured in the subsample of 1112 patients, was generally high in both groups, with fatigue, insomnia and anxiety being the most commonly reported problems. Based on the UBQ-H questionnaire, the summary utility score in survivors was 0.98 (where 0 = dead and 1 = normal good health),10 with a slight decline over time but no statistically significant difference between the pravastatin and placebo groups (Box 5). Therefore, we did not adjust life-years for quality differences.
Box 4 summarises the information on costs of resource use for the pravastatin and placebo groups, and the cost differences between the groups. The total cost difference, excluding the study medication, was $1667 per patient in favour of pravastatin. This represents a one-third cost offset against the $4913 average cost per patient of pravastatin. At the trial close, the absolute difference in all-cause mortality was 3.0% (95% CI, 1.6%–4.4%), and the net cost difference was $3246 (95% CI, $2638–$3855) per patient (Box 4). The cost per death prevented during the trial was $107 730, with a 95% CI from bootstrapping of $68 626–$209 881. A one-way sensitivity analysis on cost per life saved using 95% CIs for cost and effect factors (Box 6) indicated that the cost per life saved was most sensitive to uncertainty in absolute all-cause mortality.
The mean follow-up period was 6.0 years. Within this period, the "extra survivors" attributable to pravastatin treatment will have gained between zero and six years, with an average gain of three years. To calculate survival beyond the six-year follow-up of the trial, we constructed a life table of the 4502 patients on placebo. At the end of the study, the median age of the patients was 68 years (mean, 66.9 years), for which the within-trial life table provides a further life expectancy of 11 years (compared with the general Australian population expectation of 12.7 years for males and 16.2 for females). If the survival curves do not further diverge and no further costs are accrued, the expected cost per life-year saved (undiscounted) is $107 730/(3.0 + 11) = $7695. This simple projection assumes that the cost reductions from the healthier pravastatin group and the costs incurred by the additional survivors are roughly equal. It also ignores any additional survival benefit that may accrue among those who have avoided a cardiovascular event (eg, strokes avoided).
If future costs and benefits are both discounted (to account for their lesser value than current costs and benefits) at the standard rate for Australian cost-effectiveness analyses of 5% per year, following Pharmaceutical Benefits Advisory Committee guidelines,7 the total cost difference becomes $2943 per patient. Within the 6.0-year trial period, the discounted time gained with pravastatin was 2.7, rather than 3.0, years per extra survivor, and the within-trial life table provides a further life expectancy of 6.2 discounted years. If the survival curves do not further diverge, and no further costs are accrued, the expected cost per life-year saved (discounted at 5%) is $10 938. Considering other plausible survival effects beyond the study, if within-study treatment effects are prolonged or reversed for six years beyond the study period the discounted incremental life-years accrued would increase by roughly 50% or halve, respectively. Again, assuming no further costs are accrued, this would result in a cost per discounted-life-year saved of about $7000 for a continued treatment effect and $22 000 for reversal of the treatment effect.
The results from the LIPID study show that treatment with pravastatin in patients with established coronary heart disease and "average" cholesterol levels is effective in reducing mortality. This cost-effectiveness analysis shows that there is a cost offset of roughly a third of the total costs of pravastatin dispensed during the trial. The resultant cost-effectiveness of $107 730 per premature death prevented, or about $10 900 per discounted life-year saved, is within a range generally considered acceptable and is comparable with that of many other interventions.
Treatment was cost-effective across a number of assumptions, but was most sensitive to any prolonged treatment effect (or reversal) beyond the six years. We are therefore continuing to follow up the LIPID patients to eventually provide more reliable long-term estimates of cost-effectiveness. The results at eight years suggest that the treatment effect continues.14
How did these results compare with those of other analyses? Analyses of the cost-effectiveness of statins in cholesterol-lowering therapy are difficult to compare because of differences in the populations, healthcare practices, and cost per unit of resources. Therapy for high-risk groups, such as those with established disease and high cholesterol levels, will generally be more cost-effective because the absolute benefits will be greater and the cost offsets larger, whereas primary prevention for patients with moderate cholesterol levels and few other risk factors will be the least cost-effective. In this regard, it is interesting to compare these results with the cost-effectiveness found in the Scandinavian Simvastatin Survival Study (4S).15 That study showed a cost-effectiveness ratio that ranged from $3800 to $27 400, depending on the patient group. The studies are difficult to compare because of differences in the operation of health services in the two countries. However, the patients in 4S were at high risk, and we would therefore expect greater cost-effectiveness because of the greater absolute mortality difference. Previous modelled analysis in the United Kingdom suggested a somewhat worse cost-effectiveness than that seen in LIPID: for example, the cost-effectiveness for men aged 45–60 with a history of myocardial infarction and a cholesterol level of 5.5–6.0 mmol/L was £16 000 (discounted at 5%).16
How then can we apply this result? The absolute benefit is unlikely to be substantially influenced by country-specific factors, and, given a roughly similar price for pravastatin, the cost offsets are unlikely to change sufficiently to provide complete payment for pravastatin. Thus, at worst, the cost per life-year saved may be 50% higher than calculated (if there were no cost offsets) or may be somewhat lower depending on country-specific costings. However, this is still likely to leave it in a cost-effective range. To confirm this, modelling of country-specific cost-effectiveness is required.
The cost-effectiveness estimates do not necessarily generalise from the trial to the community. The extent of generalisability will depend on factors such as relative-risk profiles and compliance.17 However, of perhaps more importance will be modelling the cost-effectiveness within particular subpopulations to answer the question, "Which (if not all) patients with ischaemic heart disease can be treated cost-effectively with an HMG-CoA reductase inhibitor?". Subgroup analyses show similar relative-risk reductions across different age, sex, and lipid-profile groupings. Absolute risk, and hence cost-effectiveness, will therefore depend largely on individual predicted risk. Appropriate models for such risk prediction in secondary prevention are being developed, and will be used for predicting subgroup cost-effectiveness. However, in high-income countries, pravastatin is likely to be acceptably cost-effective for most patients with established coronary artery disease.
Despite the mounting evidence on the value of statin therapy, long-term therapy with statins is still underused in patients with coronary heart disease. The Australian Pharmaceutical Benefits Scheme's guidelines should be relaxed so as not to require a trial of diet therapy before statin therapy is commenced. The LIPID trial has shown that statin therapy is cost-effective when given in addition to dietary advice. The guidelines should allow statin therapy and dietary treatment to start at the same time without delay.
1: Hospitalisations, length of stay and hospital costs of patients in the LIPID study, according to treatment group*
Reason for hospitalisation |
Hospital admissions |
Average stay (days) |
Average cost per admission† ($A) |
Total cost† ($A) |
|||||||
Placebo |
Pravastatin |
Placebo |
Pravastatin |
Placebo |
Pravastatin |
Placebo |
Pravastatin |
||||
Coronary-artery bypass surgery |
498 |
405 |
14.4 |
12.2 |
12 165 |
11 209 |
6 058 346 |
4 539 444 |
|||
Unstable angina |
1 463 |
1 258 |
4.6 |
4.3 |
2 471 |
2 452 |
3 614 475 |
3 084 553 |
|||
Myocardial infarction |
450 |
338 |
8.6 |
8.6 |
4 873 |
4 941 |
2 192 656 |
1 669 896 |
|||
Angioplasty |
331 |
240 |
5.9 |
5.4 |
4 910 |
4 795 |
1 625 056 |
1 150 707 |
|||
Stroke |
140 |
113 |
18.6 |
16.1 |
7 273 |
6 608 |
1 018 157 |
746 758 |
|||
Other circulatory disorder |
2 916 |
2 617 |
5.0 |
4.5 |
3 329 |
3 130 |
9 706 152 |
8 192 416 |
|||
Diseases and disorders of the respiratory system |
796 |
713 |
6.7 |
6.9 |
3 316 |
3 285 |
2 639 317 |
2 342 095 |
|||
Diseases and disorders of the skin, subcutaneous tissue and breast |
456 |
469 |
5.5 |
3.9 |
2 570 |
1 922 |
1 171 845 |
901 634 |
|||
Diseases and disorders of the digestive system |
1 527 |
1 447 |
3.8 |
3.9 |
2 185 |
2 142 |
3 335 811 |
3 099 966 |
|||
Neoplastic disorders (haematological and solid neoplasms) |
152 |
142 |
10.6 |
6.2 |
4 678 |
3 376 |
711 020 |
479 458 |
|||
Mental diseases and disorders |
68 |
58 |
22.2 |
19.7 |
6 814 |
4 665 |
463 383 |
270 584 |
|||
Diseases and disorders of blood and blood-forming organs, and immunological disorders |
174 |
72 |
2.8 |
2.9 |
1 275 |
1 211 |
221 803 |
87 203 |
|||
Injuries, poisoning and toxic effects of drugs |
97 |
94 |
7.9 |
5.6 |
3 770 |
2 677 |
365 683 |
251 678 |
|||
Infectious and parasitic diseases (systemic, or unspecified site) |
105 |
87 |
9.1 |
6.9 |
4 935 |
4 655 |
518 175 |
404 991 |
|||
Diseases and disorders of the female reproductive system |
73 |
104 |
5.3 |
6.1 |
2 586 |
2 843 |
188 755 |
295 629 |
|||
Diseases and disorders of the musculoskeletal system and connective tissue |
872 |
894 |
7.3 |
7.2 |
5 185 |
5 220 |
4 521 607 |
4 666 249 |
|||
Diseases and disorders of the male reproductive system |
472 |
510 |
4.6 |
4.8 |
2 595 |
2 722 |
1 224 829 |
1 388 468 |
|||
Other |
2 194 |
2 148 |
5.9 |
5.1 |
3 055 |
3 054 |
6 702 061 |
6 560 544 |
|||
Total |
12 784 |
11 709 |
46 279 131 |
40 132 270 |
|||||||
* Pravastatin group: n = 4512. Placebo group: n = 4502. † Adjusted for long length-of-stay outliers according to casemix standards for NSW 1997–1998.7 |
|||||||||||
2: Costs of medication other than pravastatin, according to treatment group*
|
Drug use |
Cost ($A) |
Extra cost per person in pravastatin group ($A) |
|||||||||
Medication |
Pravastatin |
Placebo |
Pravastatin |
Placebo |
|||||||
All (non-pravastatin) cholesterol-lowering drugs |
290 427 |
1 487 466 |
–266 |
||||||||
Statins |
4 347 |
22 550 |
213 610 |
1 110 378 |
–199 |
||||||
Fibrates |
1 456 |
6 065 |
55 180 |
204 476 |
–33 |
||||||
Resins |
228 |
2 127 |
15 940 |
150 341 |
–30 |
||||||
Others |
395 |
618 |
5 697 |
22 271 |
–4 |
||||||
All cardiovascular drugs (other than cholesterol- lowering) |
7 678 605 |
7 926 424 |
–59 |
||||||||
Angiotensin-converting enzyme inhibitor |
63 998 |
65 574 |
2 070 202 |
2 121 637 |
–12 |
||||||
Antianginal drug |
116 557 |
121 091 |
2 872 871 |
2 987 187 |
–27 |
||||||
β-Blocker |
56 169 |
58 931 |
526 784 |
529 935 |
–1 |
||||||
Diuretic |
48 138 |
55 740 |
172 683 |
203 731 |
–7 |
||||||
Calcium-channel blocker |
52 994 |
55 223 |
1 198 762 |
1 249 916 |
–12 |
||||||
Other cardiac drug |
306 649 |
302 512 |
837 303 |
834 018 |
0 |
||||||
Other drugs |
2 438 697 |
2 593 381 |
–36 |
||||||||
Respiratory system drugs |
35 182 |
40 817 |
336 152 |
410 065 |
–17 |
||||||
Gastrointestinal system drugs |
31 966 |
33 958 |
912 615 |
980 709 |
–16 |
||||||
Musculoskeletal system drugs |
54 947 |
51 517 |
432 427 |
415 299 |
4 |
||||||
Psychoactive drugs |
18 145 |
19 809 |
213 048 |
240 692 |
–6 |
||||||
Others |
66 844 |
67 174 |
544 455 |
546 616 |
–1 |
||||||
Total |
10 407 729 |
12 007 271 |
–360 |
||||||||
* Pravastatin group: n = 4512. Placebo group: n = 4502. |
|||||||||||
3: Average resource use and costs associated with ambulatory care and diagnostic tests*
Item |
Average number per person† |
Average unit cost‡ ($A) |
Extra cost per person in pravastatin group§ ($A) |
||||||||
Visits to doctor in 6 weeks (n = 1112) |
|||||||||||
General practitioner |
1.217 |
26.50 |
20.67 |
||||||||
Medical specialist |
0.382 |
87.05 |
20.89 |
||||||||
Outpatient clinic |
0.280 |
17.00 |
3.23 |
||||||||
All visits to medical practitioner |
44.79 |
||||||||||
Diagnostic tests per person in 18 months (n = 485) |
|||||||||||
Cardiac investigations |
1.26 |
127.97 |
6.54 |
||||||||
Radiology |
0.78 |
73.22 |
3.16 |
||||||||
Imaging other than radiology |
0.10 |
123.50 |
0.74 |
||||||||
Pathology tests |
2.61 |
20.02 |
2.15 |
||||||||
Tests other than pathology |
0.12 |
123.60 |
0.62 |
||||||||
All tests |
13.20 |
||||||||||
* Based on substudies of patients assessed at 1, 3 and 5 years after randomisation. Diagnostic tests were reported over the previous 6 months, doctor visits over the previous 2 weeks. † Average number of visits or tests per person summed over the 3 sample periods for combined pravastatin and placebo groups (P > 0.10 in all cases). ‡ Average unit cost of each visit and test. § Extra costs attributable to greater survival in pravastatin arm over the 6.0 years of the study, with common average resource use per person applied. |
|||||||||||
4: Summary of resource costs and cost differences between pravastatin and placebo groups
Total costs in each group ($A) |
|||||||||||
Resource |
Pravastatin (n = 4512) |
Placebo (n = 4502) |
Extra cost per person in pravastatin group* ($A) |
||||||||
Hospital use |
40 132 270 |
46 279 131 |
–1385 |
||||||||
Cardiovascular* |
19 524 071 |
24 360 241 |
–1084 |
||||||||
Other than cardiovascular |
20 608 198 |
21 918 890 |
–301 |
||||||||
Medications |
10 407 729 |
12 007 273 |
–360 |
||||||||
Cholesterol-lowering |
290 427 |
1 487 466 |
–266 |
||||||||
Other cardiovascular |
7 678 605 |
7 926 424 |
–59 |
||||||||
Other |
2 438 697 |
2 593 381 |
–36 |
||||||||
Ambulatory care |
17 109 639 |
16 871 056 |
45 |
||||||||
Diagnostic tests |
5 011 405 |
4 940 859 |
13 |
||||||||
Nursing home |
783 450 |
689 449 |
21 |
||||||||
Total costs other than pravastatin |
73 444 493 |
80 787 768 |
–1667 |
||||||||
Pravastatin for study |
22 166 895 |
0 |
4913 |
||||||||
Total |
95 611 388 |
80 787 768 |
3246 |
||||||||
* Includes all diseases and disorders of the circulatory system, plus heart transplant and stroke. |
|||||||||||
5: Utility scores* on the quality-of-life questionnaire in a subsample of 1112 patients over five years, by treatment group
Group |
Baseline |
Year 1 |
Year 3 |
Year 5 |
|||||||
Pravastatin (n = 555) |
0.983 |
0.981 |
0.981 |
0.978 |
|||||||
Placebo (n = 557) |
0.982 |
0.983 |
0.980 |
0.976 |
|||||||
* On a scale from 0 = dead to 1 = normal good health. |
|||||||||||
6: Sensitivity of incremental cost per life saved to 95% CIs of costs and survival effects
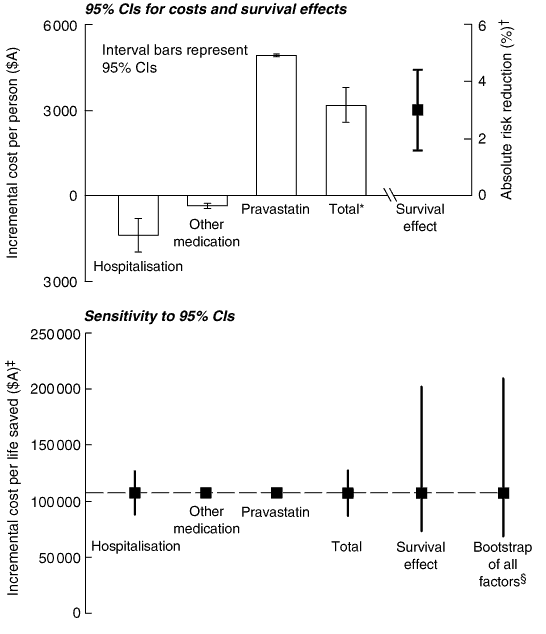
* Includes an incremental fixed cost of $79 for outpatient diagnostic test and nursing home costs. † Absolute risk reduction for all-cause mortality. ‡On a baseline of $107 730. § The bootstrapped 95% CI for incremental cost per life saved included uncertainty for all survival effects, and hospitalisation, pravastatin and other medication costs.
Received 12 December 2001, accepted 23 July 2002
- Paul P Glasziou1
- Simon D Eckermann2
- Sarah E Mulray3
- R John Simes4
- Andrew J Martin
- Adrienne C Kirby6
- Susan Caleo7
- Jane P Hall8
- Harvey D White9
- Andrew M Tonkin10
- 1 School of Population Health, University of Queensland, Brisbane, QLD.
- 2 NHMRC Clinical Trials Centre, University of Sydney, Camperdown, NSW.
- 3 Centre for Health Economics Research and Evaluation (CHERE), Camperdown, NSW.
- 4 Coronary Care and Cardiovascular Research Unit, Green Lane Hospital, Auckland, New Zealand.
- 5 National Heart Foundation of Australia, Melbourne, VIC.
We wish to thank Margot Mellies (Bristol-Myers Squibb) and Joe Jackson (Bristol-Myers Squibb) for encouragement, Cam Donaldson (University of Calgary, Canada), Peter Davey (Medical Technology Assessment Group, Sydney, NSW), Virginia Wiseman (Centre for Health Economics Research and Evaluation, Sydney, NSW), and Mike Aristides (Medical Technology Assessment Group, Sydney, NSW) for economic advice, Manjula Schou (NHMRC Clinical Trials Centre, Sydney, NSW) for statistical help, the 85 LIPID centres, and the LIPID trial patients.
Some members of the LIPID Management Committee have received honoraria for invited lectures about the LIPID study in general, or have benefited indirectly from a research grant to the University of Sydney.
- 1. Hunink M, Goldman L, Tosteson ANA, et al. The recent decline in mortality from coronary heart disease, 1980–1990. The effect of secular trends in risk factors and treatment. JAMA 1997; 277: 535-542.
- 2. The Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344: 1383-1389.
- 3. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996; 335: 1001-1009.
- 4. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339: 1349-1357.
- 5. Henry D. Economic analysis as an aid to subsidisation decisions: the development of Australian guidelines for pharmaceuticals. Pharmacoeconomics 1992; 1: 54-67.
- 6. Glasziou PP, Simes RJ, Hall J, Donaldson C. Design of a cost effectiveness study within a randomized trial: the LIPID trial for secondary prevention of IHD. Control Clin Trials 1997; 18: 464-476.
- 7. Guidelines for the pharmaceutical industry on preparation of submissions to the Pharmaceutical Benefits Advisory Committee. Canberra: Commonwealth Department of Health and Ageing, Mar 1999. Available at <http://www.health.gov.au/internet/wcms/publishing.nsf/Content/health-pbs-general-pubs-pharmpac-gusubpac.htm> Accessed Jun 2002, link updated Oct 2005.
- 8. Commonwealth of Australia. Manual of resource items and their associated costs. Canberra: AGPS, 1992: 1-24.
- 9. Commonwealth Department of Health and Ageing. Medical benefits schedule (MBS). Available at <http://www.health.gov.au/pubs/mbs/>
- 10. Commonwealth Department of Health and Ageing. Schedule of pharmaceutical benefits for approved pharmacists and medical practitioners. Available at <http://www1.health.gov.au/pbs/>
- 11. Martin A, Glasziou PP, Simes RJ. A cardiovascular extension of the health measurement questionnaire. J Epidemiol Community Health 1999; 53: 548-557.
- 12. Martin A, Glasziou PP, Simes RJ, Lumley T. Predicting patient's utilities from quality of life items: an improved scoring system for the UBQ-H. Qual Life Res 1998; 7: 703-711.
- 13. Briggs A, O'Brien B, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost effectiveness studies. Annu Rev Public Health 2002; 23: 377-401.
- 14. LIPID Study Group. Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: the LIPID trial follow-up. Lancet 2002; 359: 1379-1387.
- 15. Johannesson M, Jonsson B, Kjekshus J, et al. Cost effectiveness of simvastatin treatment to lower cholesterol in patients with coronary heart disease. N Engl J Med 1997; 336: 332-336.
- 16. Pharoah PDP, Hollingworth W. Cost effectiveness of lowering cholesterol concentration with statins in patients with and without pre-existing coronary heart disease: life table method applied to health authority population. BMJ 1996; 312: 1443-1448.
- 17. Hughes DA, Bagust A, Haycox A, Walley T. The impact of non-compliance on the cost-effectiveness of pharmaceuticals: a review of the literature. Health Econ 2001; 10: 601-615.





Abstract
Objective: To measure the cost-effectiveness of cholesterol-lowering therapy with pravastatin in patients with established ischaemic heart disease and average baseline cholesterol levels.
Design: Prospective economic evaluation within a double-blind randomised trial (Long-Term Intervention with Pravastatin in Ischaemic Disease [LIPID]), in which patients with a history of unstable angina or previous myocardial infarction were randomised to receive 40 mg of pravastatin daily or matching placebo.
Patients and setting: 9014 patients aged 35–75 years from 85 centres in Australia and New Zealand, recruited from June 1990 to December 1992.
Main outcome measures: Cost per death averted, cost per life-year gained, and cost per quality-adjusted life-year gained, calculated from measures of hospitalisations, medication use, outpatient visits, and quality of life.
Results: The LIPID trial showed a 22% relative reduction in all-cause mortality (P < 0.001). Over a mean follow-up of 6 years, hospital admissions for coronary heart disease and coronary revascularisation were reduced by about 20%. Over this period, pravastatin cost $A4913 per patient, but reduced total hospitalisation costs by $A1385 per patient and other long-term medication costs by $A360 per patient. In a subsample of patients, average quality of life was 0.98 (where 0 = dead and 1 = normal good health); the treatment groups were not significantly different. The absolute reduction in all-cause mortality was 3.0% (95% CI, 1.6%–4.4%), and the incremental cost was $3246 per patient, resulting in a cost per life saved of $107 730 (95% CI, $68 626–$209 881) within the study period. Extrapolating long-term survival from the placebo group, the undiscounted cost per life-year saved was $7695 (and $10 938 with costs and life-years discounted at an annual rate of 5%).
Conclusions: Pravastatin therapy for patients with a history of myocardial infarction or unstable angina and average cholesterol levels reduces all-cause mortality and appears cost effective compared with accepted treatments in high-income countries.