Over millions of years, evolution has selected for darker skin pigmentation at low latitudes with high sun exposure, whereas lighter pigmentation may have been favoured at higher latitudes to enhance cutaneous synthesis of vitamin D.1 However, in the past 300 years, many human groups have defied this evolutionary selection and migrated rapidly into new areas — too recently to have adapted naturally to the environment. Thus, we now see high rates of skin cancer in lightly pigmented populations displaced to areas of high ambient ultraviolet radiation (UVR) and vitamin D deficiency disorders in deeply pigmented people who have moved to places of high latitude.
In addition, in the past 50 years, European Australians have shed their Victorian prudishness and donned less and less clothing — a suntan has become fashionable for some, and summertime clothing has shrunk to a minimum. Despite medical knowledge of the adverse effects of excessive UVR on health, a recent survey showed that the proportion of students aged 12–17 years reporting at least one episode of sunburn in the previous summer had risen from 68% in 1993 to 80% in 1999.2 But, for at least some of the UVR-related diseases, it is likely that it is these years of carefree sun exposure that may contribute most to the development of diseases such as melanoma, basal-cell carcinoma3 and cataract in later life.
It is inappropriate to eschew all exposure to UVR. There are diseases related to insufficient as well as excessive UVR exposure, and the burden of disease related to UVR is likely to be a U-shaped, not linear, curve (Box 1).
It has proved difficult to formulate quantitative radiation-based dose–response relationships for UVR exposure and disease, even when the causal relationship is clear. Epidemiological studies must rely on recalled experience of sun exposure or markers of personal sun exposure (such as actinic damage to the skin) as measures of past UVR exposure. Moreover, it is likely that for some diseases there is not a simple linear relationship between UVR exposure and disease — the timing and pattern of excess exposure may be crucial.3
It is not possible to directly equate physical measures of ambient UVR to doses of personal UVR exposure: at any latitude, for any level of ambient UVR, there may be a 100-fold difference in personal UVR exposure that is related to behavioural factors.4 In addition, differences in host pigmentation substantially alter the dose–response relationship for both beneficial and deleterious effects. Deeply pigmented skin is estimated to have a minimal erythemal dose 33-fold higher than the fairest skin, with resultant low rates of UVR-related skin cancer.5 Put in other terms, deeply pigmented skin is estimated to have a sun protection factor of 13.4, almost equivalent to a fair-skinned person constantly wearing SPF 15 sunscreen applied to the whole body.6 On the other hand, the UVR dose required for vitamin D synthesis increases substantially with increasing skin pigmentation — a sixfold increase in exposure time for very black skin,7 and a twofold increase for Asian skin,8 compared with fair skin.
There is a considerable burden of disease in Australia caused by excess exposure to UVR, particularly in lightly pigmented populations who are not adapted, in an evolutionary sense, to high ambient UVR levels. In 1999, cutaneous malignant melanoma was listed in the 25 leading causes of disease burden in Australia, ahead of stomach cancer, schizophrenia and leukaemia.9 At least 90% of melanoma is probably attributable to excessive UVR exposure.10 Add to this the calculated disease burden for non-melanoma skin cancers, and it is clear that excessive exposure to UVR causes a significant disease burden.
A recent assessment of the global burden of disease caused by UVR6 extended this calculation to include other diseases thought to be directly attributable to excess UVR exposure: sunburn, reactivation of herpes labialis, photo-ageing, cortical cataract, pterygium and squamous-cell carcinoma of the cornea and conjunctiva.
For most diseases caused by excessive UVR exposure, there is a long lag period between exposure and disease, or disease is caused by a gradual accumulation of exposure experience.3 Unfortunately, cessation of exposure later in life may only partially reduce the risk of disease incurred by heavy exposure earlier in life.3 Patterns of sun-seeking behaviour in our teenagers today are likely to be reflected in disease incidence 20–30 years from now, and the steady rise in incidence of non-melanoma skin cancers in Australia over the past 20 years11 may relate to a similar rise in sun-loving behaviour in the previous 20-year period.
Apart from the well known effects of UVR exposure on skin and eyes, recent research indicates that UVR suppresses components of both local and systemic immune function.12 In particular, UVR appears to dampen T-helper type 1 (TH1) lymphocyte immune activity. UVR-induced immune suppression may work both through promoting higher levels of vitamin D, which appears to have immunosuppressive effects, and through other pathways that do not include vitamin D.12
Adverse effects of such UVR-induced immunosuppression might include reduction in host resistance to infection and a decrease in the effectiveness of some vaccines, particularly those for tuberculosis, measles and hepatitis.13 Such effects have been confirmed in animal studies,14 and researchers are investigating the possible effects (adverse or beneficial) of UVR-induced immunosuppression in humans. For example, a recent randomised controlled trial has shown that narrow-band UVB therapy can significantly reduce human atopic eczema, possibly through subepidermal immune suppression.15
Over 90% of vitamin D is produced endogenously from the action of UVB on precursors in the skin.16 A small burden of disease is related to frank vitamin D deficiency disorders (rickets, osteomalacia and osteoporosis) caused by underexposure to UVR. For other diseases, such as multiple sclerosis, type 1 diabetes and rheumatoid arthritis, a possible causal relationship with UVR exposure requires further substantiation, and their effect on disease burden is yet to be determined.6
It is increasingly being recognised that many Australians may have insufficient sun exposure to allow adequate synthesis of cutaneous precursors to vitamin D — either because of behavioural practices or because their level of skin pigmentation requires a longer UVR exposure to produce a given amount of vitamin D.7 Elderly or institutionalised people, and infants of mothers with low levels of vitamin D, are most at risk of resultant muscle and bone diseases.7,17 Recent population-based studies in Geelong (latitude 38ºS) and southeast Queensland (latitude 27ºS) have shown that vitamin D insufficiency and vitamin D deficiency are common, even in healthy populations of European descent, in regions with moderate levels of ambient UVR.17,18
Modern urban populations spend large amounts of time, including leisure time, indoors. Health promotion must tread a fine line between advice against excessive UVR exposure and advice against excessive sun avoidance. In particular, public health messages regarding sun avoidance to decrease skin cancer should be carefully evaluated to ensure that vitamin D deficiency disorders do not increase.
Recent research suggests that vitamin D and sunlight may be important to human health in ways that are unrelated to their effects on bone.16,19-21 Many tissues have vitamin D receptors, and the active form of vitamin D may have important roles in immunomodulation and regulation of tumour-cell proliferation, differentiation and spread.19 Thus, vitamin D deficiency may have a role in the aetiology of various cancers (eg, of the prostate, breast and colon),19 as well as in schizophrenia (due to effects of low prenatal vitamin D on the developing brain)20 and a number of immune disorders (eg, type 1 diabetes mellitus and multiple sclerosis [MS]).12
The prevalence of childhood type 1 diabetes mellitus increases with increasing latitude and decreases with infant vitamin D supplementation.21,22 There is also a gradient of increasing MS with increasing latitude. In Australia, the absolute correlation with annual average ambient UVR levels is stronger for MS prevalence than for malignant melanoma incidence.23 The variation in MS prevalence with UVR levels could reflect differential UVR-induced immune suppression of autoimmune activity.24 In particular, the autoimmune profile of MS is characterised by disturbances of those T-cell-related activities that are specifically affected by UVB.24
The epidemiological features of some of these TH1-mediated autoimmune diseases are partially consistent with a protective effect for high personal UVR exposure, but the data are not conclusive. Further research is required to examine this issue, as well as the effect of insufficient sunlight on mood,25 seasonal affective disorder26 and the sleep/wake cycle.27
The wide gap between the UVR dose that is required to synthesise sufficient vitamin D cutaneously and the higher UVR dose that will cause skin and eye disorders is often not appreciated. For example, exposure of 6%–10% of the body surface (face, hands and arms) to sunlight at noon in Boston (42°N) in spring, summer and autumn for five minutes two to three times a week is thought to be sufficient to maintain vitamin D levels in a person with a moderately fair complexion.28 In contrast, at a higher ambient UVR location, Geraldton, Western Australia (29°S), a person with maximal risk for basal-cell carcinoma may have been receiving as much as 14 hours of sun exposure per week for every week of life.29 For any individual, it should be possible to obtain the beneficial effects of adequate vitamin D levels from appropriate UVR exposure, without risking skin and eye disease from excess exposure.
UVR is absorbed by stratospheric ozone in its path through the atmosphere (Box 2). Maximal absorption of UVR occurs in the short-wavelength UVC band and minimal absorption in the longer UVA band. Most UVB is absorbed by stratospheric ozone, but about 10% reaches the lower atmosphere,6 where tropospheric ozone, pollution or cloud cover may further attenuate the amount of UVB radiation reaching ground level. While UVA is implicated in the aetiology of some disorders, it appears that it is UVB that has particular effects on cutaneous DNA and other cutaneous chromophores (light-absorbing molecules) — and, through these, local and systemic effects on the immune system and tumour genesis.5
Over time, humans have developed technology to make harsh climates apparently safer and more hospitable. In the 1920s, remarkable new compounds such as the chlorofluorocarbons (CFCs) were developed that could be used in refrigeration and as spraycan propellants. These compounds seemed very stable and safe, unlike the ammonia that they replaced. Little did we suspect that their use would have serious consequences for the level of ozone in our atmosphere (Box 3).
In the late 1960s and early 1970s the possibility of stratospheric ozone depletion caused by human activities (exhaust gases from supersonic aircraft and then use of CFCs) was raised.30 The role of stratospheric ozone as a filter for UVB radiation was known, and rising levels of skin cancer were being attributed to exposure to UVR, probably UVB.30 In 1978, the United States banned the use of CFCs in aerosols, and in early 1985 the Vienna Convention for the Protection of the Ozone Layer represented international concern about the state of the ozone layer and consequent risks to health. Later that year, routine atmospheric testing in the Antarctic revealed extremely low levels of atmospheric ozone.30 This Antarctic spring "ozone hole" has become a common occurrence and is believed to be caused by catalytic destruction of stratospheric ozone by CFC byproducts. In 1987, building on the intentions outlined in the Vienna Convention, the Montreal Protocol called for a 50% reduction of five important ozone-depleting chemicals by the end of 1999.31 The 1992 Copenhagen Amendments to this protocol recommended that the production of 21 ozone-depleting chemicals be reduced to zero by the end of 1995.31
Stratospheric ozone levels vary markedly day to day, season to season, but the trend has been for levels to be fairly stable from 1926 to 1973, with a fall in ozone levels between 1973 and the late 1990s (Box 3). There is recent evidence of an increase in ambient, ground-level UVB with decreasing levels of stratospheric ozone (Box 3).4
It is clear that there is a substantial disease burden associated with both excessive exposure to UVR and inadequate exposure to UVR. It is not within the scope of this review to make guidelines regarding appropriate levels of UVR exposure. However, we offer the following suggestions:
If patients are likely to have low personal UVR exposure, doctors should encourage a diet high in vitamin D, monitor serum vitamin D levels and, if low, consider vitamin D supplementation.
Excessive exposure to UVR should continue to be discouraged. If individuals need to be in the direct sun in high ambient UVR conditions, then doctors should strongly encourage sun-protection measures. However, we need to make sure this message is not taken to the opposite extreme of reducing beneficial UVR exposure, such as short exposures during winter.
Public health messages regarding sun exposure should be tailored to the population — with regard to levels of pigmentation, behavioural practices, and regional and seasonal levels of ambient UVR.
While stratospheric ozone depletion may increase ground-level UVB, and thus the potential for increased total UVR exposure, human behaviour with respect to personal sun exposure and dietary intake of vitamin D may be the most important determinant of the disease burden related to UVR in the future (Box 4).
1: Schematic diagram of the relation between ultraviolet radiation (UVR) exposure and the burden of disease
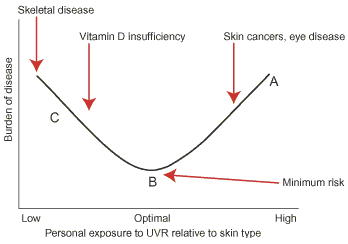
Points A and C represent inappropriate UVR exposure. Europeans in Australia with high outdoor UVR exposure typify point A. Point C represents people with insufficient UVR exposure, whose dietary vitamin D intake will also be important in determining their vitamin D status. Point B represents optimal UVR exposure: a person with careful titration of correct UVR dose for skin type.
2: The electromagnetic spectrum and penetration of ultraviolet radiation through the Earth's atmosphere*
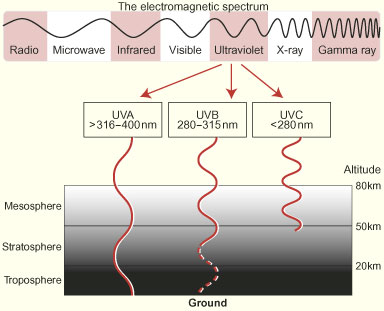
*Adapted from Office of Public Outreach, Space Telescope Science Institute. Amazing space. Available at: <http://amazing-space.stsci.edu/light/ems-frames.html>.
3: Stratospheric ozone levels and changes in the ultraviolet (UV) index over time
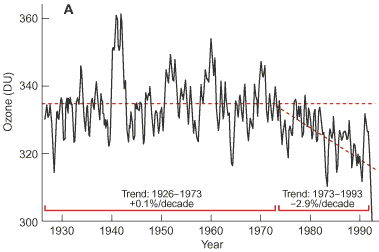
A: Variation in ozone levels 1926–1993 at Arosa, Switzerland (one-year-smoothed data). Courtesy of NASA. SEES: Studying Earth's environment from space. June 2000. Available at <http://see.gsfc.nasa.gov/edu/SEES>.
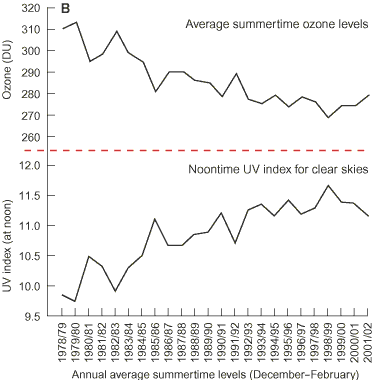
B: Variations in stratospheric ozone and UV index at Lauder, Central Otago, New Zealand, 1978–2002. A clear overall decrease in ozone and increase in UV radiation can be seen over the period 1980–2000. There is a suggestion of a recovery since then, but the time is too short to detect it unambiguously over the natural variability (Richard McKenzie, National Institute of Water and Atmospheric Research, Lauder, NZ, personal communication).
DU = Dobson units.
4: Reflections and predictions
Circa 1900
Consequences
Public health advice
|  Photo courtesy of the Talk.Origins Archive. Available at: http://www.talkorigins.org/faqs/homs/rickets.html |
|
Possible scenarios, circa 2100 Scenario one (assuming adherence to Copenhagen Amendments to Montreal Protocol)
Scenario two (assuming adherence to unamended Montreal Protocol only)
Scenario three (based on behavioural changes only)
Public health advice Scenarios one and two: Sophisticated UVR exposure messages, taking into account regional and seasonal differences in ambient UVR and differing host pigmentation. Wearing of personal UVR monitor when outdoors recommended. Scenario three: Sun avoidance encouraged; vitamin D supplementation recommended. | |
- Robyn M Lucas1
- Anne-Louise Ponsonby2
- National Centre for Epidemiology and Population Health, Australian National University, Canberra, ACT.
We thank our colleagues, particularly B Armstrong, T Dwyer and A McMichael, for helpful discussions on these issues; E Slatyer for graphical assistance; and S Amerikow for assistance with reviewing the literature.
None identified.
- 1. Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol 2000; 39: 57-106.
- 2. Lemon R. Taming the trend: are teenage girls still seeking that flawless tan "to die for"? Healthlink 2002; Autumn: 9.
- 3. Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B 2001; 63: 8-18.
- 4. Gies P, Roy C, McLennan A, Tomlinson D. Trends in ultraviolet radiation. Trends in sun protection seminar. Melbourne: Anti-Cancer Council of Victoria, 1999.
- 5. Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol 2001; 79: 547-568.
- 6. Lucas RM, McMichael AJ, Smith WT, Armstrong BK. Comparative risk assessment: ultraviolet radiation. World Health Organization 2003. In press.
- 7. Mason RS, Diamond TH. Vitamin D deficiency and multicultural Australia. Med J Aust 2001; 175: 236-237.
- 8. Lo CW, Paris PW, Holick MF. Indian and Pakistani immigrants have the same capacity as Caucasians to produce vitamin D in response to ultraviolet irradiation. Am J Clin Nutr 1986; 44: 683-685.
- 9. Mathers C, Vos T, Stevenson C. The burden of disease and injury in Australia. Canberra: Australian Institute of Health and Welfare, 1999: 245.
- 10. Armstrong BK, Kricker A. How much melanoma is caused by sun exposure? Melanoma Res 1993; 3: 395-401.
- 11. Staples M, Marks R, Giles G. Trends in the incidence of non-melanocytic skin cancer (NMSC) treated in Australia 1985-1995: are primary prevention programs starting to have an effect? Int J Cancer 1998; 78: 144-148.
- 12. Ponsonby A-L, McMichael AJ, van der Mei I. Ultraviolet radiation and autoimmune disease: insights from epidemiological research. Toxicology 2002. In press.
- 13. Selgrade MK, Repacholi MH, Koren HS. Ultraviolet radiation-induced immune modulation: potential consequences for infectious, allergic, and autoimmune disease. Environ Health Perspect 1997; 105: 332-334.
- 14. Norval M, Garssen J, Van Loveren H, el-Ghorr AA. UV-induced changes in the immune response to microbial infections in human subjects and animal models. J Epidemiol 1999; 9(6 Suppl): S84-S92.
- 15. Reynolds N, Franklin V, Gray JC, et al. Narrow-band ultraviolet B and broad-band ultraviolet A phototherapy in adult atopic eczema: a randomised controlled trial. Lancet 2001; 357: 2012-2016.
- 16. Norris JM. Can the sunshine vitamin shed light on type 1 diabetes? Lancet 2001; 358: 1476-1478.
- 17. Pasco JA, Henry MJ, Nicholson GC, et al. Vitamin D status of women in the Geelong Osteoporosis Study: association with diet and casual exposure to sunlight. Med J Aust 2001; 175: 401-405.
- 18. McGrath JJ, Kimlin MG, Saha S, et al. Vitamin D insufficiency in south-east Queensland. Med J Aust 2001; 174 : 150-151.
- 19. Studzinski GP, Moore DC. Sunlight — can it prevent as well as cause cancer? Cancer Res 1995; 55: 4014-4022.
- 20. McGrath J. Hypothesis: is low prenatal vitamin D a risk-modifying factor for schizophrenia? Schizophr Res 1999; 40: 173-177.
- 21. Hypponen E, Laara E, Reunanen A, et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 2001; 358: 1500-1503.
- 22. Eurodiab substudy 2 study group. Vitamin D supplement in early childhood and risk for type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1999; 42: 51-54.
- 23. van der Mei IA, Ponsonby A-L, Blizzard L, Dwyer T. Regional variation in multiple sclerosis prevalence in Australia and its association with ambient ultraviolet radiation. Neuroepidemiology 2001; 20: 168-174.
- 24. McMichael AJ, Hall AJ. Does immunosuppressive ultraviolet radiation explain the latitude gradient for multiple sclerosis? Epidemiology 1997; 8: 642-645.
- 25. Ness AR, Frankel SJ, Gunnel DJ, Davey Smith G. Are we really dying for a tan? BMJ 1999; 319: 114-116.
- 26. Mersch PP, Middendorp HM, Bouhuys AL, et al. Seasonal affective disorder and latitude: a review of the literature. J Affect Disord 1999; 53: 35-48.
- 27. Dijk DJ, Lockley SW. Integration of human sleep–wake regulation and circadian rhythmicity. J Appl Physiol 2002; 92: 852-862.
- 28. Holick MF. Sunlight "D"ilemma: risk of skin cancer or bone disease and muscle weakness. Lancet 2001; 357: 4-6.
- 29. Kricker A, Armstrong BK, English DR, Heenan PJ. A dose–response curve for sun exposure and basal cell carcinoma. Int J Cancer 1995; 60: 482-488.
- 30. Morrisette PM. The evolution of policy responses to stratospheric ozone depletion. Natural Resources Journal 1989; 29: 793-820.
- 31. Slaper H, Velders GJM, Daniel JS, et al. Estimates of ozone depletion and skin cancer incidence to examine the Vienna Convention achievements. Nature 1996; 384: 256-258.





Abstract
While excessive exposure to ultraviolet radiation (UVR) is a significant cause of disease burden in Australia and the Western Pacific region, there are well documented beneficial as well as adverse effects of UVR exposure.
Ambient UVR levels do not translate directly to personal UVR dose and thus to biological effect — each person's sun-exposure behaviour and pigmentation also play a role.
Exposure in childhood may be more important than exposure in adulthood for both beneficial and adverse effects.
Stratospheric ozone depletion increases ambient UVR in the UVB wavelength, possibly the most important wavelength for both beneficial and deleterious health effects.
There is ongoing research examining the effects of UVR exposure on immune function, including an examination of the possible role of lack of UVR exposure in the aetiology of multiple sclerosis and type 1 diabetes mellitus.