The toxic components of some jellyfish venoms are sensitive to heat. For example, 15 minutes' exposure to a temperature of 60ºC markedly decreases the activity of venom from bluebottles (Physalia spp.).1 The venom from Chironex fleckeri, a large box jellyfish (pictured above), is also sensitive to temperature, with decreased activity after prolonged exposure (days to months) to temperatures between –10ºC and 5ºC,2,3 and complete loss of activity after much shorter exposure (minutes) to 45ºC.3
Nematocysts (stinging cells) were extracted from tentacles of mature specimens of C. fleckeri and lyophilised, as described by Bloom et al.4 Nematocysts were then ruptured with a bead mill beater to release venom.5 Venom concentration in the resulting extract was assumed to be correlated with protein concentration,6 which was determined by a Bradford Lowry assay.5
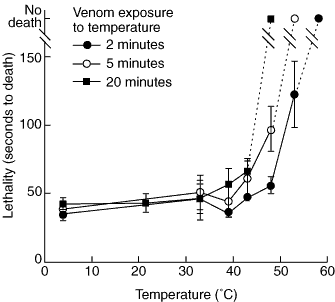
Aliquots of extracted venom were placed at temperatures of 4°C, 21.5°C, 33°C, 39°C, 43°C, 48°C, 53°C or 58°C (a range which includes and exceeds the temperatures reported as affecting box jellyfish venom).3 Three replicates were placed at each temperature for periods of two, five or 20 minutes, then returned to an ice bath for cooling. Two minutes corresponds to the approximate observed time of death of prey in the field, while 20 minutes corresponds to the time to onset of systemic symptoms in Irukandji syndrome.7
Venom lethality was determined by measuring the time to cardiac standstill after injection of venom into freshwater crayfish (Cherax quadricarinatus) (Box 1A). Cardiac standstill was defined as a period of 10 seconds without a heart beat, determined by vascular Doppler ultrasound (Box 1B). If cardiac standstill had not occurred after 10 minutes, the crayfish were observed in holding tanks over 24 hours to ensure death did not occur.
Venom lethality was affected significantly by both temperature (F7,34 = 21915; P < 0.0001) and time of exposure (F2,34 = 9907; P < 0.0001). For temperatures between 4ºC and 39ºC, post-hoc analysis revealed no significant difference in mean time to death of crayfish regardless of time of exposure. However, at temperatures of 43ºC and above, venom lost its lethality more rapidly the longer the exposure time (Box 2). Venom exposed to a temperature of 48ºC for 20 minutes failed to cause death in any experimental animals. At 50ºC, five minutes' exposure was needed for the same effect, and at 53ºC two minutes' exposure.
This experiment shows that exposing extracted C. fleckeri venom to temperatures above 39ºC dramatically affects its lethality. The effect of temperature depends on time of exposure, with higher temperatures reducing lethality in much shorter times.
These data have implications for extraction and handling of C. fleckeri venom. They may also have implications for treating jellyfish stings, although the potential for using heat clinically for C. fleckeri envenoming is limited. Firstly, these stings can cause death within minutes,7 and secondly heat may cause vasodilation and enhance movement of venom into the circulatory system.
However, heat application may be of benefit in stings by other box jellyfish, where venom distribution and the development of systemic effects appear to be slower. For instance, in Irukandji syndrome (caused by some tropical carybdeids), systemic effects appear up to 20–40 minutes after the sting.7 If the venom of these jellyfish has similar lability to C. fleckeri venom and could be contained within an area and treated with heat, it might be denatured before further symptoms develop. Nevertheless, when the sting is minor and un-noticed and systemic symptoms have already developed, application of heat may be ineffective.
Heat application is currently used to treat and provide pain relief in stonefish envenomings, with suggestions that the site be submerged in water at about 43ºC.7 However, as pain recurs when the site is removed from the water, it appears that the venom is not deactivated. Heat packs and hot showers also appear to aid patients envenomed by the Hawaiian carybdeid Carybdea alata by decreasing perceived pain,8-10 although again this may not mean that venom is deactivated.
While the temperatures needed to deactivate box jellyfish venom may render heat impractical for general treatment, the potential for further research in this area is clear.
- Teresa J Carrette1
- Jamie E Seymour2
- Paul Cullen3
- Peter L Peiera4
- Mark Little5
- 1 Tropical Biology, James Cook University – Cairns Campus, Cairns, QLD, Australia.
- 2 Department of Emergency Medicine, Cairns Base Hospital, Cairns, QLD, Australia.
- 3 Department of Emergency Medicine, Sir Charles Gairdner Hospital, Perth, WA, Australia.
None identified.
- 1. Bucherl W, Buckley EE. Venomous animals and their venom. Volume 3. Venomous invertebrates. New York: Academic Press, 1971; 417.
- 2. Endean R, Duchemin C, McColm D, Fraser H. A study of the biological activity of toxic material derived from nematocysts of the cubomedusan Chironex fleckeri. Toxicon 1969; 6: 179-204.
- 3. Endean R. Separation of two myotoxins from nematocysts of the Box Jellyfish Chironex fleckeri. Toxicon 1987; 25: 483-492.
- 4. Bloom DA, Burnett JW, Alderslade P. Partial purification of Box Jellyfish (Chironex fleckeri) nematocyst venom isolated at the beachside. Toxicon 1998; 36: 1075-1085.
- 5. Carrette TJ. An investigation into the extraction and ecology of venom in two species of cubozoans, Chironex fleckeri and Chiropsalmus sp. [MSc thesis]. Cairns: James Cook University, 2002.
- 6. Bloom DA, Radwan FFY, Burnett JW. Toxinological and immunological studies of capillary electrophoresis fractionated Chrysaora quinquecirrha (Desor) fishing tentacle and Chironex fleckeri Southcott nematocyst venoms. Comp Biochem Physiol C Toxicol and Pharmacol 2001; 128: 75-90.
- 7. Williamson JA, Fenner PJ, Burnett JW, Rifkin JF, editors. Venomous and poisonous marine animals — a medical and biological handbook. Sydney: University of New South Wales Press, 1996.
- 8. Burnett JW. Medical aspects of jellyfish envenomation: pathogenesis, case reporting and therapy. Hydrobiologia 2001; 45: 1-9.
- 9. Yoshimoto CM, Yanagihara AA. Cnidarian (coelenterate) envenomations in Hawaii improve following heat application. Trans R Soc Trop Med Hyg 2002; 96: 300-303.
- 10. Thomas CS, Scott SA, Galanis DJ, Goto RS. Box jellyfish (Carybdea alata) in Waikiki. Their influx cycle plus the analgesic effect of hot and cold packs on their stings to swimmers at the beach: a randomized, placebo controlled, clinical trial. Hawaii Med J 2001; 60: 100-107.







Abstract
Objective: To determine the effect of temperature on lethality of venom from Chironex fleckeri (the potentially fatal box jellyfish).
Design: Venom extracted from nematocysts of mature Chironex fleckeri specimens was exposed to temperatures between 4°C and 58°C for periods of two, five or 20 minutes, and then injected into freshwater crayfish (Cherax quadricarinatus) to assess lethality.
Main outcome measure: Venom lethality, assessed as time to cardiac standstill in crayfish after intramuscular injection.
Results: Venom lethality was significantly affected by both temperature (F7,34 = 21915; P < 0.0001) and time of exposure (F2,34 = 9907; P < 0.0001). No significant loss of lethality was seen after exposure to temperatures ≤ 39°C, even after 20 minutes' exposure. At temperatures ≥ 43°C, venom lost its lethality more rapidly the longer the exposure time. Venom was non-lethal after exposure to 48°C for 20 minutes, 53°C for five minutes, and 58°C for two minutes.Conclusion: Exposure to heat dramatically reduces the lethality of extracted C. fleckeri venom. Although heat application may be of limited use in treating C. fleckeri envenoming because of the speed of symptom onset, its use in other box-jellyfish envenomings, such as Irukandji syndrome, requires investigation.