Non-sedating antihistamines are available as "over-the-counter" preparations and have been widely promoted in public advertisements. However, two such antihistamines, terfenadine and astemizole, have been withdrawn in Australia and overseas. Their use was associated with ventricular arrhythmias — QT-interval prolongation on the surface electrocardiogram (ECG) and polymorphic ventricular tachycardia ("torsade de pointes").1-3
We report a case of ventricular tachycardia (VT) which was probably "torsade de pointes" following ingestion of a single tablet of loratadine.
A 43-year-old woman presented after her identical twin died suddenly, presumably from cardiac arrhythmia. The patient was asymptomatic, had no other risk factors and was not taking any regular medication. Clinical examination showed evidence of mitral valve prolapse, with normal left ventricular function and trivial mitral regurgitation on echocardiography. Several ECGs (Box 1a) and her biochemical profile were normal. An electrophysiological study did not show inducible ventricular arrhythmias with standard stimulation testing or adrenaline provocation. Atrial fibrillation was induced during catheter introduction into the right atrium, with ventricular rate less than 130/minute. A prophylactic automatic implantable defibrillator (Medtronic Micro Jewel 7221) was subsequently inserted. Transient prolongation of the QT interval was noted on ECG monitoring in association with non-sustained VT (Box 1b) within 48 hours of the implant.
Two years later, the patient had an episode of presyncope, interrupted by a spontaneous defibrillator shock, about 90 minutes after taking a single 10 mg tablet of loratadine for minor symptoms of nasal congestion; this was the patient's first exposure to this drug. She was reviewed on the following day, when the defibrillator device history revealed a rapid ventricular rhythm with changing axis of the intracardiac ventricular electrogram, with a cycle length of 320–230 ms (average rate, 250/minute; Box 2), successfully terminated by a 31-Joule biphasic discharge. The patient was not taking other medications at the time.
The term "torsade de pointes" describes polymorphic ventricular tachycardia where the QRS axis appears to be twisting around a baseline. It is associated with prolongation of the QT interval, usually greater than 500 ms (normal is less than 440 ms). A pattern of long–short cycle length in the beats immediately before tachycardia is typical of torsade de pointes (Box 3). It is only possible to diagnose probable "torsade de pointes" in our case, as the defibrillator placed in the patient recorded only a brief snapshot after the device had made the diagnosis of a treatable arryhthmia, so the initiating beats were not recorded. Further, the tracing that the device makes is a ventricular electrocardiogram without any atrial tracing, and the intracardiac QT is not generally accepted as a valid measure of the QT interval. However, the rate of the arrhythmia and its twisting morphology, together with the previous brief episode of prolonged QT interval, make any other diagnosis unlikely.
If we could have made a definite diagnosis, this would be the first documented case of torsade de pointes following ingestion of the non-sedating antihistamine loratadine. In this patient's case, the family history of sudden death and the prior history of transient QT-interval prolongation suggests a possible congenital predisposition to the deleterious effects of a drug with potential to prolong the QT interval. Prolongation of the QT interval and torsade de pointes can result from a variety of drug therapies. The likelihood of developing deleterious cardiac reactions to drugs that can prolong the QT interval can be enhanced under certain conditions (Box 4).4-6
Two non-sedating antihistamines (terfenadine and astemizole) were withdrawn after reports of sudden cardiac death.7,8 They were found to block the channels that regulate entry of potassium into cardiac cells during the repolarisation phase of the action potential. Blockade of these channels produces lengthening of the action potential duration.1,2 This is expressed as QT prolongation on ECG and is associated with the development of early after-depolarisations which can give rise to polymorphic VT and, in turn, degenerate into ventricular fibrillation.
Loratadine has been retained because of favourable laboratory and clinical data.7 Although cardiac deaths have been reported in relation to this drug,3 no life-threatening arrhythmias have been documented. However, a mechanism for susceptibility to ventricular arrhythmias is suggested by a recent report demonstrating blockade of the human ether-a-go-go-related potassium channel in a similar manner to terfenadine in an animal preparation.9
This case suggests the need for caution in administering loratadine in patients with a predisposition to arrhythmias. Patients should be cautioned to report symptoms such as palpitations, presyncope or syncope after exposure to this drug. As QT-interval prolongation may be intermittent, routine ECG screening may not accurately identify susceptible patients.
1: Electrocardiograms from the patient before (a) and after (b) implantation of an automatic implantable defibrillator
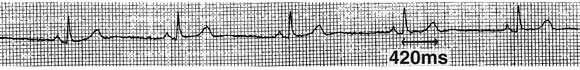
(a) ECG strip showing sinus bradycardia (rate, 50/min) and a normal QT interval (420 ms).
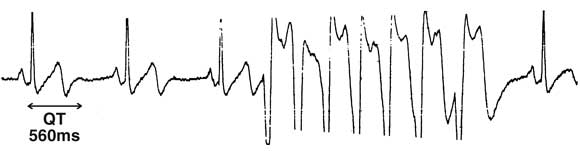
(b) ECG monitor strip showing QT prolongation (560 ms) and a run of non-sustained ventricular tachycardia (arrow).
2: Intracardiac electrogram obtained following spontaneous discharge of the implanted defibrillator
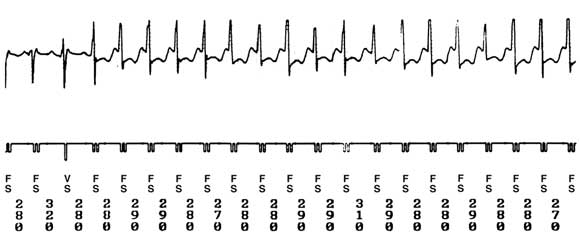
VS = ventricular sensed beat; FS = ventricular beat within the fibrillation zone. Numbers at the bottom denote the tachycardia cycle length in milliseconds.
3: Electrocardiogram rhythm strip illustrating torsade des pointes (not from this patient)

ECG shows classical torsade de pointes with long QT interval (a), long–short initiating cycles and twisting QRS complexes (b).
4: Factors predisposing to QT-interval prolongation
Electrolyte abnormalities: hypokalaemia, hypocalcaemia, hypomagnesaemia
Metabolic disturbance: hypothyroidism, hypothermia, anorexia
Drugs: class I and class III antiarrhythmics, antihistamines (eg, astemizole, terfenadine), psychiatric drugs (eg, tricyclic antidepressants, haloperidol, lithium, prochlorperazine, sertindole), antimicrobials (eg, macrolides, ketoconazole, chloroquine, halofantrine), cisapride
Structural heart disease: left ventricular hypertrophy or failure
Severe renal or hepatic dysfunction
Congenital long-QT syndrome
- 1. De Abajo FJ, Rodriguez LAG. Risk of ventricular arrhythmias associated with nonsedating antihistamine drugs. Br J Clin Pharmacol 1999; 47: 307-313.
- 2. Monahan BP, Ferguson CL, Killeavy ES, et al. Torsade de pointes occurring in association with terfenadine use. JAMA 1990; 264: 2788-2790.
- 3. Stratmann HG, Kennedy HL. Torsade de pointes associated with drugs and toxins: recognition and management. Am Heart J 1987; 113: 1481.
- 4. Zehender M, Hohnloser S, Just H. QT-interval prolonging drugs: mechanisms and clinical relevance of their arrhythmogenic hazards. Cardiovasc Drug Ther 1991; 5: 515-530.
- 5. Zipes DP. Proarrhythmic effects of antiarrhythmic drugs. Am J Cardiol 1987; 59: 26E-31E.
- 6. Haverkamp W, Breithardt G, Camm AJ, et al. The potential for QT prolongation and proarrhythmia by non-anti-arrhythmic drugs: clinical and regulatory implications. Report on a policy conference of the European Society of Cardiology. Cardiovasc Res 2000; 47: 219-233.
- 7. Lindquist M, Edwards IR. Risks of non-sedating antihistamines. Lancet 1997; 349: 1322.
- 8. Routledge PA, Lindquist M, Edwards IR. Spontaneous reporting of suspected adverse reactions to antihistamines: a national and international perspective. Clin Exp Allergy. 1999; 29 Suppl 3: 240-246.
- 9. Crumb WJ. Loratadine blockade of K+ channels in human heart: comparison with terfenadine under physiological conditions. J Pharm Exp Ther 2000; 292: 261-264.





None declared.