Interest in the quality of prescribing has been driven by three main forces: concerns about drug costs,1 the adverse effects of prescription drug use in the community,2 and the desire to ensure that the results of large clinical trials are integrated into clinical practice.
Ensuring that prescribing practices are in line with the best evidence on efficacy and safety requires objective information to enable audit and review of current practices. Feedback of prescribing data to practitioners has been introduced in several countries including Australia.3-9 Data for these programs are usually derived from administrative systems established to enable prescription payments. A common prescribing "indicator" is the volume of prescribing by a doctor, often expressed as a prescribing rate, where numbers of prescriptions are related to numbers of patients or consultations. In most feedback programs (including that of the Australian Health Insurance Commission), the individual is compared with the average performance of her or his peers, which may not reflect best practice. Some authors have suggested the need to move beyond measures of prescribing volume and to identify clinically relevant indicators that reflect prescribing quality.10 A key requirement of these indicators is the availability of valid and reliable data for their derivation.
The aim of this study was to determine whether it is possible to use prescribing data collected routinely by the Health Insurance Commission (HIC) to generate meaningful prescribing indicators for use in general practice. HIC prescribing data are incomplete, and we were particularly interested in the effects of this on the validity of the prescribing indicators.
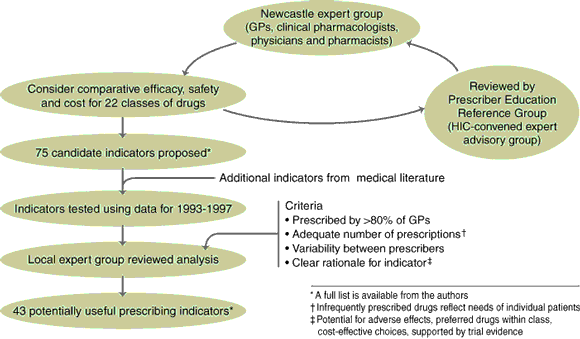
We used two expert groups which sequentially and systematically reviewed each of 22 classes of drugs available on the Pharmaceutical Benefits Scheme (PBS), identifying issues of comparative efficacy, safety and cost that might be reflected in prescribing indicators. One group, convened specifically for this project, comprised general practitioners, specialist physicians, clinical pharmacologists and pharmacists from the Newcastle area and drug utilisation experts from Canberra. The second group, the Prescriber Education Reference Group (an expert group convened by the HIC to advise on a broad range of prescribing-related activities), reviewed the work of the first group, offering further expert input and critical comment. The indicators identified for testing reflected a range of issues, including high overall levels of use of individual drugs and classes of drugs, relative use of agents within and between classes of drugs (on the basis of safety and efficacy), and the use of a limited number of agents within a drug class. Additional prescribing indicators identified through a literature search were also included in this study3,11-13 (see Box 1).
The HIC data collection has been described elsewhere.14 Records are generated when the government contributes to the cost of a medical service provided under Medicare or a pharmaceutical product dispensed under the PBS. Almost all services provided by GPs generate a record in the Medicare services file.
For pharmaceuticals, there is a series of graded copayments, with a maximum patient contribution of $20.00 (in 1997) for general beneficiaries and $3.20 for concession card holders (mostly pensioners and other social security beneficiaries). When the patient pays the entire cost of the medication, there is no HIC record of the prescription.
Many commonly prescribed drugs (eg, NSAIDs, anti-infective agents, diuretics, β-blockers, benzodiazepines and tricyclic antidepressants) cost less than $20.00, so only prescriptions of these drugs to concession card holders are recorded by the HIC. During the study period, there was complete capture for more expensive drugs (including statins, proton pump inhibitors and H2-receptor antagonists). Between 1993 and 1997, the maximum patient copayment per item rose from $15.90 to $20.00, resulting in a number of drugs changing from complete to incomplete data capture.
Prescriptions recorded by the HIC cannot be attributed to individual patients, so there is no information on the age or sex of the patient, co-prescribed medications, or the indication for treatment.
Analyses were restricted to GPs providing ≥ 1500 Medicare services per year. This workload stratification has been used in other studies,15 and excludes GPs whose prescribing profiles are likely to be unreliable because of small numbers of prescriptions. Prescribing and Medicare services data from the HIC for the years 1993 to 1997 were used. Analyses were based on original prescriptions, as these represent opportunities for prescribing decisions by GPs (ie, to start, stop or continue treatment), and because the identity of the prescriber was poorly recorded for repeat prescriptions (authorised on the prescription form at the same time as the prescribing decision) in HIC datasets in the earlier years of analysis.
Drug-prescribing records for individual GPs were extracted by the HIC and summarised. Records were identified only by a recoded provider number to prevent identification of individual GPs. Prescribing rates were defined as the numbers of original prescriptions for a drug or drug class dispensed per 100 Medicare services provided by the GP.6 Ratio and proportional indicators were used to estimate the relative use of agents within a drug class, or the relative use of different classes of drugs by the GP.
Indicator data were stratified according to the completeness of data collection in the HIC files: "complete", "incomplete" or "changing" data capture between 1993 and 1997. The influence of incomplete capture was explored by stratifying GPs according to the proportions of the practice patients who were concession card holders. The higher the proportion of concession card holders, the greater the degree of prescribing data capture for that GP.
Temporal trends in the prescribing indicators were compared with national trends in estimated prescription numbers obtained from Australian Statistics on Medicines (ASM).16 ASM data comprise prescription totals derived from HIC data files and an estimate of use of "under-copayment" drugs and drugs purchased with a private prescription. These estimates are derived from a statistically representative sample of Australian pharmacies. The two datasets are not directly comparable: the prescribing indicators were calculated for individual GPs and were derived from HIC records of original prescriptions, whereas data in ASM are aggregated across all practitioners (GPs and specialists) by drug, and include original and repeat prescriptions. ASM utilisation data are population adjusted and expressed as numbers of Defined Daily Doses (DDDs)/1000 inhabitants/day.17 Despite their differences, the trends in the two datasets should be similar. PBS prescription packs generally provide one month of treatment (or a standard course of treatment), so numbers of prescriptions should relate to DDD estimates.
The expert groups identified 75 prescribing indicators worthy of further analysis (the full list is available from the authors). The statistical properties of these (and other indicators identified in the literature) were examined using 1993–1997 data for approximately 14 000 GPs providing ≥ 1500 Medicare services per year (ranging from 13 206 in 1993 to 15 425 in 1997). Four indicators reflected the use of personal formularies (based on the use of a limited number of agents within a drug class) and have been reported elsewhere.19 Of the remainder, 43 prescribing indicators were identified which were thought to reflect the quality of prescribing and to be informative for GPs and regional or national authorities (Box 2).
Thirty-one indicators were prescribing rates. Sixteen applied to drugs or groups of drugs where high prescribing rates were believed to be suboptimal. These related to adverse effects: antibiotics (resistance);20,21 flucloxacillin and amoxycillin–clavulanic acid (hepatotoxicity);22 prochlorperazine (drug-induced parkinsonism and tardive dyskinesia);23 non-steroidal anti-inflammatory drugs (NSAIDs) (gastrointestinal and cardiac effects);24-26 benzodiazepines (dependence);27 or where the benefits of therapy may be outweighed by the risks of serious adverse effects (eg, cisapride28). Two prescribing rate indicators involved drugs where a relatively high rate of prescribing could be interpreted as desirable in the absence of additional clinical information (phenoxymethylpenicillin and trimethoprim). There were various reasons for the other 13 indicators. For example, some were drugs of interest because they were comparatively new and expensive, others had been rapidly taken up into prescribing and there was evidence of more cost-effective choices, and single-drug products were generally preferred to combination drugs.
Twelve indicators were ratios, reflecting relative use within (or between) classes of drugs. Concerns related to adverse effects: long-acting sulfonylureas may be associated with hypoglycaemic episodes, particularly in the elderly,29 and NSAIDs with low to medium risk of gastrointestinal adverse effects are preferred over high-risk agents24,25 (the study was conducted before the introduction of COX-2 inhibitors). Others were based on a lack of evidence of additional benefits or clinical superiority to justify the increased cost of therapy (indapamide versus cheaper thiazide diuretics;29 nebuliser therapy over other metered-dose inhalers with spacer devices30).
There was substantial variability between prescribers. This is illustrated in Box 3 for 17 indicators, where there were fivefold to more than 300-fold differences between GPs' prescribing. Generally, the variation in ratio indicators was greater than that seen for prescribing rates.
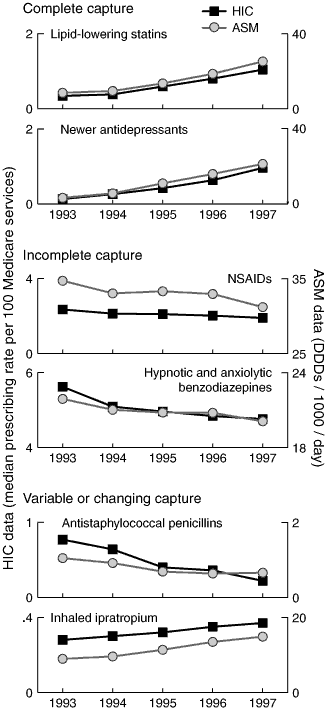
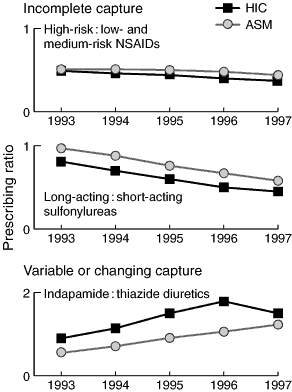
Data capture was complete for six indicators, consistently incomplete for 20 indicators and variable for 17 indicators (Box 2).
Trends in estimated prescribing rates were similar to those in national aggregate data from ASM for drugs for which HIC data capture was consistently complete or incomplete. However, when data capture varied, indicators based on HIC data alone provided a misleading picture of prescribing trends (Box 4). The apparent decrease in prescribing of antistaphylococcal penicillins from 1996 to 1997 concealed a change in extent of capture of flucloxacillin prescribing data in 1997. An increase from one to two inhaler units per prescription in 1995 explains some of the observed differences in prescribing trends for ipratropium.
None of the ratio indicators involved drugs for which there was complete HIC data capture for both numerator and denominator. When data capture was consistently incomplete, ratios were broadly similar to the crude ratios derived from aggregate data from ASM (Box 5). The two estimates differed when data capture changed over time. The discrepant trends in the ratio of indapamide to thiazide diuretics between 1996 and 1997 can be explained in part by a change from complete to incomplete data capture for indapamide in 1996.
For drugs with incomplete capture (NSAIDs, benzodiazepines), there were fivefold to sevenfold differences in median prescribing rates across the five strata defined by the proportion of concession card holders in the practice (Box 6). In comparison, differences were twofold to threefold for drugs with complete capture (statins, newer antidepressants).
Ratio indicators for drugs with incomplete capture were less affected (Box 6). Estimates for the ratios of high-risk to low- + medium-risk NSAIDs and long-acting to short-acting sulfonylureas varied only slightly across the GP strata (Box 6). This was also true for estimates of the ratio of indapamide to thiazides in 1997. However, there was more variability across the strata in estimates for 1996, when there was complete capture of indapamide prescriptions and incomplete capture for thiazide diuretics (Box 6).
The prescribing indicators used in this study were based on consensus rather than evidence. Nevertheless, the measures recommended for further testing have face validity, reflect important clinical or cost-effectiveness issues, and can be derived from aggregate prescribing data. Despite the systematic approach taken by the two expert groups to review all 22 major drug classes, and the large size of the HIC files, only a small number of prescribing indicators covering a narrow range of prescribing practices were identified as potentially useful. Few of these could be interpreted unambiguously without access to additional clinical information. For example, assessing the appropriateness of prescribing decisions for angiotensin-converting enzyme inhibitors, calcium-channel blockers or lipid-lowering statins requires clinical information that is not available from HIC data.
Of equal concern is the effect of incomplete data capture (where prescribing costs are below the levels of the general patient copayment) on the interpretation of the prescribing indicators. For instance, in the case of NSAIDs, the apparent rates of prescribing by GPs with the highest proportions of concession card holders were five times higher than those of GPs with the lowest proportion of card holders among their patients. The data for drugs with complete data capture indicate that about half of this range is due to artefact, while the other half might reflect real differences in the needs of the populations, based on age, disease severity and comorbidity. The main danger of using flawed indicators is that doctors might have profiles that appear suboptimal, but are confounded by the socioeconomic profile of their patient population.
Ratio indicators (which show choice of medication once a decision to prescribe has been made) were less affected by incomplete data capture (Box 6). However, the ratio measures had more extreme values and more variability among prescribers, which might make them difficult to interpret.
Changes in the extent of data capture over time introduced measurement artefacts that affected interpretation of prescribing trends. There is no gold standard against which to test the indicators; however, we assumed that ASM data provide the best estimates of overall trends in prescribing. We expected that HIC data, however expressed, should provide a similar picture of prescribing over time. However, trends in both prescribing rate and ratio indicators were misleading when there were changes in the extent of HIC data capture (Box 4 and Box 5).
There are attractions in deriving prescribing indicators from routinely collected HIC data. Data collection, analysis and feedback can be performed cheaply. However, the data will never satisfy the requirement that GPs should only be compared with other GPs practising in similar settings, seeing similar numbers of patients with similar clinical conditions.
To achieve a satisfactory degree of validity and reliability, prescribing indicators need to be based on all prescriptions, irrespective of whether they attract a Commonwealth payment. This information is available on the computers of community pharmacists across the country, but is inaccessible for routine monitoring purposes. The identification of valid, reliable, credible and clinically relevant prescribing indicators is in its infancy. Prescribers themselves need to become involved in their development and interpretation. A key requirement is valid, complete and reliable data to support their derivation.
2: Potentially useful prescribing indicators identified by the expert groups (extent of data capture)
Prescribing rate indicators (n = 31) |
Prescribing ratio indicators (n = 12) |
||||||||||
Antibacterial agents* Quinolones (complete†) Paediatric‡ antibacterial agents (incomplete) Trimethoprim–sulfamethoxazole (incomplete) Trimethoprim (incomplete) Cephalosporins (incomplete) Macrolides (incomplete) Phenoxymethylpenicillin (incomplete) Oral antibacterial agents (variable) Antistaphylococcal penicillins (variable) Tetracyclines (variable) |
Antibacterial agents (Paediatric‡) Amoxycillin–clavulanic acid : amoxycillin (incomplete) Roxithromycin : erythromycin (incomplete) Trimethoprim–sulfamethoxazole: trimethoprim (incomplete) Amoxycillin–clavulanic acid : amoxycillin (variable) |
||||||||||
Gastrointestinal drugs H2-receptor antagonists (complete) Proton pump inhibitors (complete) Cisapride tablets (complete) |
Endocrine drugs Long-acting sulfonylureas : short-acting sulfonylureas (incomplete) |
||||||||||
Central nervous system drugs Newer antidepressants (complete) Hypnosedative benzodiazepines (incomplete) Anxiolytic benzodiazepines (incomplete) Hypnosedative and anxiolytic benzodiazepines combined§ (incomplete) Prochlorperazine tablets (incomplete) Tricyclic antidepressants (incomplete) |
|||||||||||
Musculoskeletal drugs Non-steroidal anti-inflammatory drugs (incomplete) |
Musculoskeletal drugs (High-risk NSAIDs¶) : (low- + medium-risk NSAIDs**) (incomplete) Allopurinol 300 mg : allopurinol 100 mg (incomplete) |
||||||||||
Cardiovascular drugs Lipid-lowering statins (complete) Thiazide diuretics (incomplete) Combination diuretics (incomplete) Angiotensin-converting enzyme inhibitors (variable) Calcium-channel blockers (variable) Diuretics overall (variable) Indapamide (variable) β-Blockers (variable) |
Cardiovascular drugs Indapamide : thiazide diuretics (variable) (ACE inhibitors + calcium channel blockers) : (diuretics + β-blockers) (variable) Isosorbide mononitrate : isosorbide dinitrate (variable) |
||||||||||
Respiratory drugs Inhaled bronchodilators (variable) Inhaled glucocorticoids (variable) Inhaled ipratropium (variable) |
Respiratory drugs Nebuliser : other inhaled dosage forms (all drugs) (variable) Nebuliser : other inhaled dosage forms (ipratropium) (variable) |
||||||||||
* Unless otherwise specified, indicators for antibacterial agents are based on prescriptions for oral tablet and capsule formulations. |
|||||||||||
3: Variability in indicators for individual GPs' prescribing (1997)
Indicator |
Median |
Range* |
Ratio of highest to lowest† |
||||||||
Prescribing rates‡ |
|||||||||||
H2-receptor antagonists |
2.24 |
0.61–5.24 |
9 |
||||||||
Proton pump inhibitors |
0.30 |
0.05–0.97 |
19 |
||||||||
Cisapride (tablets) |
0.17 |
0.03–0.68 |
23 |
||||||||
Non-steroidal anti-inflammatory drugs |
1.90 |
0.47–4.64 |
10 |
||||||||
Hypnosedative benzodiazepines |
2.56 |
0.40–8.79 |
22 |
||||||||
Anxiolytic benzodiazepines |
2.04 |
0.29–7.64 |
26 |
||||||||
Hypnosedative and anxiolytic benzodiazepines |
4.76 |
0.80–15.91 |
20 |
||||||||
Prochlorperazine (tablets) |
0.52 |
0.09–1.85 |
21 |
||||||||
Antistaphylococcal penicillins |
0.22 |
0.03–0.92 |
31 |
||||||||
Oral antibacterial agents |
6.60 |
2.52–12.44 |
5 |
||||||||
Ratios |
|||||||||||
Amoxycillin–clavulanic acid : amoxycillin |
0.56 |
0.06–4.57 |
76 |
||||||||
(Paediatric) Amoxycillin–clavulanic acid : amoxycillin |
0.20 |
0–2.57 |
> 250 |
||||||||
Indapamide : thiazide diuretics |
1.50 |
0.05–12.00 |
240 |
||||||||
High-risk NSAIDs : low- + medium-risk NSAIDs |
0.37 |
0.08–1.42 |
18 |
||||||||
Long-acting : short-acting sulfonylureas |
0.45 |
0–3.00 |
> 300 |
||||||||
Nebuliser : other inhaled dosage forms (all drugs) |
0.34 |
0.09–0.86 |
10 |
||||||||
Nebuliser : other inhaled dosage forms (ipratropium) |
1.36 |
0.13–8.00 |
62 |
||||||||
* Range is the values between the 5th and 95th percentiles of the prescribing indicator. |
|||||||||||
4: Temporal trends for prescribing rate indicators: Health Insurance Commission (HIC) versus Australian Statistics on Medicines (ASM) data
5: Temporal trends for prescribing ratio indicators: Health Insurance Commission (HIC) versus Australian Statistics on Medicines (ASM) data
6: Effects of incomplete data capture on prescribing rates and ratios (1997)
Median prescribing rate per 100 Medicare services
Proportion of concession patients (number of GPs) |
Incomplete data capture |
Complete data capture |
|||||||||
NSAIDs |
Benzodiazepines* |
Statins |
Newer antidepressants |
||||||||
0–19% (2722) |
0.75 |
1.58 |
0.52 |
0.66 |
|||||||
20%–29% (4016) |
1.44 |
3.37 |
0.81 |
0.87 |
|||||||
30%–39% (4110) |
2.19 |
5.58 |
1.15 |
1.05 |
|||||||
40%–49% (2934) |
2.88 |
7.93 |
1.44 |
1.14 |
|||||||
≥ 50% (1643) |
3.66 |
10.6 |
1.63 |
1.17 |
|||||||
All GPs |
1.90 |
4.76 |
1.04 |
0.96 |
|||||||
Median prescribing ratio
Proportion of concession patients (number of GPs) |
Incomplete data capture |
Changing data capture |
|||
NSAIDs† |
Sulfonylureas‡ |
Indapamide: thiazides§ (1996) |
Indapamide: thiazides (1997) |
||
0–19% (2722) |
0.32 |
0.33 |
2.00 |
1.33 |
|
20%–29% (4016) |
0.35 |
0.43 |
2.00 |
1.50 |
|
30%–39% (4110) |
0.39 |
0.45 |
1.75 |
1.56 |
|
40%–49% (2934) |
0.39 |
0.50 |
1.64 |
1.54 |
|
≥ 50% (1643) |
0.41 |
0.48 |
1.50 |
1.61 |
|
All GPs |
0.37 |
0.45 |
1.79 |
1.50 |
|
* Hypnosedative and anxiolytic benzodiazepines combined (diazepam, oxazepam, nitrazepam, temazepam).
† Ratio (high-risk NSAIDs) : (low- + medium-risk NSAIDs).
‡ Ratio (long-acting sulfonlyureas) : (short-acting sulfonylureas).
§ The numbers of GPs in each stratum were smaller for this analysis (1857, 3485, 3841, 2813 and 1542 GPs).
Received 9 August 2001, accepted 30 December 2001
- Jane Robertson1
- Jayne L Fryer2
- Dianne L O'Connell3
- Anthony J Smith4
- David A Henry5
- Department of Clinical Pharmacology, School of Medical Practice and Population Health, Faculty of Health, University of Newcastle, Newcastle, NSW.
None declared.
- 1. PBS expenditure and prescriptions April 2000 to March 2001. Canberra: Department of Health and Aged Care, 2001. Available at <http://www.health.gov.au/pbs/pubs/pbbexp/pbmar01/index.htm> (no longer available - related pages at http://www.health.gov.au/internet/wcms/publishing.nsf/Content/Pharmaceutical+Benefits+Scheme+%28PBS%29-3).
- 2. Roughead EE. The nature and extent of drug-related hospitalisations in Australia. J Qual Clin Pract 1999; 19: 19-22.
- 3. Bateman DN, Soutter J, Eccles M, et al. Setting standards of prescribing performance in primary care: use of a consensus group of general practitioners and application of standards to practices in the north of England. Br J Gen Pract 1996; 46: 20-25.
- 4. Prescribing Support Unit. Prescribing measures and their application. An explanation. Leeds: Prescribing Support Unit, 1998.
- 5. Malcolm L, Wright L, Seers M, Guthrie J. An evaluation of pharmaceutical management and budget holding in Pegasus Medical Group. N Z Med J 1999; 112: 162-164.
- 6. O'Connell DL, Henry D, Tomlins R. Randomised controlled trial of effect of feedback on general practitioners' prescribing in Australia. BMJ 1999; 318: 507-511.
- 7. Von Ferber L, Bausch J, Köster I, et al. Pharmacotherapeutic circles. Results of an 18-month peer-review prescribing-improvement programme for general practitioners. Pharmacoeconomics 1999; 16: 273-283.
- 8. McGavock H, Wilson-Davis K, Niblock RWF. Unsuspected patterns of drug utilization revealed by interrogation of a regional general practitioner prescribing database. Pharmacoepidemiol Drug Safety 1992; 1: 73-80.
- 9. Veninga CC, Denig P, Zwaagstra R, Haaijer-Ruskamp FM. Improving drug treatment in general practice. J Clin Epidemiol 2000; 53: 762-772.
- 10. McColl A, Roderick P, Gabbay J, et al. Performance indicators for primary care groups: an evidence based approach. BMJ 1998; 317: 1354-1360.
- 11. Avery AJ, Heron T, Lloyd D, et al. Investigating relationships between a range of potential indicators of general practice prescribing: an observational study. J Clin Pharm Ther 1998; 23: 441-450.
- 12. Baker SJ. Use of performance indicators for general practice. BMJ 1996; 312: 58.
- 13. Campbell SM, Cantrill JA, Roberts D. Prescribing indicators for UK general practice: Delphi consultation study. BMJ 2000; 321: 1-5.
- 14. Edmonds DJ, Dumbrell DM, Primrose JG, et al. Development of an Australian drug utilisation database. Pharmacoeconomics 1993; 3: 427-432.
- 15. Bridges-Webb C, Britt H, Miles DA, et al. Morbidity and treatment in general practice in Australia 1990–1991. Med J Aust 1992; 157 Suppl Oct 19: S1-S57.
- 16. Commonwealth Department of Health and Aged Care. Australian Statistics on Medicines 1998. Canberra: AusInfo, 1999.
- 17. Merlo J, Wessling A, Melander A. Comparison of dose standard units for drug utilisation studies. Eur J Clin Pharmacol 1996; 50: 27-30.
- 18. SAS Institute Inc. SAS procedures guide, version 6. 3rd ed. Cary, NC: SAS Institute Inc, 1990.
- 19. Robertson J, Fryer JL, O'Connell DL, et al. Personal formularies. An index of prescribing quality? Eur J Clin Pharmacol 2001; 57: 333-341.
- 20. Magee JT, Pritchard EL, Fitzgerald KA, et al on behalf of the Welsh Antibiotic Study Group. Antibiotic prescribing and antibiotic resistance in community practice: retrospective study, 1996–8. BMJ 1999; 319: 1239-1240.
- 21. Turnidge J. What can be done about resistance to antibiotics? BMJ 1998; 317: 645-647.
- 22. David M. Hepatic disorders. In: Davies DM, Ferner RE, de Glanville H, editors. Davies's textbook of adverse drug reactions. 5th ed. London: Chapman & Hall Medical, 1998; 288-289.
- 23. Seymour RA. Oral and dental disorders. In: Davies DM, Ferner RE and de Glanville H, editors. Davies's textbook of adverse drug reactions. 5th ed. London: Chapman & Hall Medical, 1998; 247-248.
- 24. Henry D, Lim LL-Y, Garcia Rodriguez LA, et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. BMJ 1996; 312: 1563-1566.
- 25. Hernández-Diaz S, Garcia Rodriguez LA. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation. An overview of epidemiologic studies published in the 1990s. Arch Intern Med 2000; 160: 2093-2099.
- 26. Page J, Henry D. Consumption of NSAIDs and the development of congestive cardiac failure in elderly patients: an underrecognized public health problem. Arch Intern Med 2000; 160: 777-784.
- 27. Möller H-J. Effectiveness and safety of benzodiazepines. J Clin Psychopharmacol 1999; 19 Suppl 2: 2S-11S.
- 28. Adverse Drug Reactions Advisory Committee (ADRAC). Restriction of indications for cisapride. ADRAC Bulletin 2000; 19 (4).
- 29. Australian medicines handbook. 2nd ed. Adelaide: Australian Medicines Handbook Pty Ltd, 2000.
- 30. Cates CJ, Rowe BH. Holding chambers versus nebulisers for beta-agonist treatment of acute asthma [Cochrane review]. In: The Cochrane Library, Issue 2, 2001. Oxford: Update Software.





Abstract
Objectives: To derive indicators of quality prescribing by Australian general practitioners based on Health Insurance Commission (HIC) data and assess the influence of incomplete capture of data on under-copayment drugs on the validity of these indicators.
Design: Two expert groups proposed prescribing indicators that can be derived from aggregate prescribing data, and which reflect important clinical or cost-effectiveness issues. Indicators were examined using HIC data and compared with national prescribing trends over time using Australian Statistics on Medicines. The effect of incomplete data capture on indicator interpretation was examined by stratifying GPs into five strata based on the proportion of concession card holders in their practice.
Participants: Approximately 14 000 Australian GPs providing ≥ 1500 Medicare services per year.
Main outcome measures: Measures of prescribing for individual GPs (based on HIC data 1993–1997).
Results: Forty-three potentially useful indicators were identified. These covered a fairly narrow range of prescribing activities and many required additional clinical information for interpretation. Indicators based on prescribing rates gave a misleading picture of prescribing trends where the extent of HIC data capture changed over time. Indicators expressed as ratios that reflected choice of agent within a drug class were less affected by incomplete data capture.
Conclusions: Indicators of quality prescribing can be derived from HIC data. However, indicators for under-copayment drugs that represent prescribing rates may unfairly classify doctors practising in areas of socioeconomic disadvantage or high morbidity as "high prescribers". Ratio indicators are more robust, and may be more valid prescribing measures. If HIC data are to be used to monitor the quality of prescribing, data on all prescriptions dispensed will be needed.