Seventy per cent of children treated for childhood malignancy survive into adulthood.1 This improved survival is accompanied by long term complications. Some oncology centres have long term surveillance clinics, but, increasingly, these survivors will be followed by general practitioners, who may not be aware of their patients' health risks.
Radiation-induced thyroid neoplasia in children is a recognised result of direct irradiation (eg, Hodgkin's disease),2,3 and of the obsolete practice of radiotherapy for benign childhood conditions.4,5 The Chernobyl reactor disaster also demonstrated the tumorigenic effect of both ingested and inhaled radioactive isotopes in childhood, with a more than 30-fold increase in the number of cases of childhood thyroid cancer.6,7
The young thyroid is vulnerable to the tumorigenic effects of radiation, and shows a linear response from 0.1 Gy levelling at 10 Gy; the excess risk persists for up to four decades.8 Although radiation therapy is now used infrequently, a large cohort of irradiated survivors remain at lifelong risk of thyroid neoplasia.
We describe the thyroid status of consecutive survivors of childhood cancer attending a follow-up clinic for survivors of childhood malignancy who had received thyroid irradiation. The data are used to formulate a practical surveillance plan directed at primary healthcare service providers.
The Late Effects Oncology Clinic (LEC) is a multispecialty clinic within the Oncology Unit of the Children's Hospital, Westmead. The Oncology Unit treats about 120 new patients each year. Patients attend from all over New South Wales and are enrolled at the LEC at least five years after completion of their cancer treatment.
Patients had been irradiated using megavoltage photons (cobalt-60 or 4–6 MV linear accelerators).
The thyroid doses were estimated from a retrospective review of radiation treatment data, including diagrams, photographs and simulation films. The thyroid dose received during prophylactic cranial irradiation was estimated using previously described techniques.9
Because of the wide range of doses, analysis was based on the estimated dose received by individuals rather than a group. For the purposes of analysis, dose was treated as a continuous variable.
Microsoft Excel10 and SPSS for Windows11 were used for the analysis. Results are reported as odds ratios (OR) with 95% confidence intervals, or standard deviations where appropriate. Statistical significance was accepted at P < 0.05.
A multivariate logistic regression analysis was used to examine the relation between prognostic factors and the incidence of malignancy. Factors examined included age at original diagnosis, sex, thyroid palpability, time since irradiation, radiation dose of the thyroid, nodule type (none, single, multiple), and nodal involvement.
The study population comprised 142 subjects (70 girls). Thirty-five patients eligible to attend were not enrolled in the LEC; these patients did not differ from the entire LEC or current study group in diagnostic category or cancer therapy. The subjects' mean age was 16.0 years, and the mean age at the commencement of original therapy was 5.8 years. The mean time since completion of therapy for the study group was 14.0 years.
Treatments resulting in direct irradiation included localised treatment of lymphomas and sarcomas following chemotherapy, wide-field irradiation of lymphomas, total-body irradiation (TBI) before bone marrow transplantation, and the exit dose from the spinal component of craniospinal irradiation.
The main treatment resulting in scatter radiation to the thyroid was cranial radiation used for central nervous system prophylaxis in haematological malignancies. Other conditions for which the thyroid had received scatter radiation were sarcomas and cerebral tumours.
Some patients had received more than one course of radiation (eg, cranial irradiation followed by TBI). These individuals were considered to have received a direct dose.
Box 1 shows the main original diagnoses in the two groups. The median thyroid dose in the direct group was 20 Gy (range, 5–50 Gy). The dose was fractionated, with the exception of several patients who received the entire dose as a single fraction of TBI (7–10 Gy). The doses received by scatter were estimated at 0.1–0.5 Gy in multiple fractions.
Twenty-eight patients were taking thyroxine at the time of the study (25 for treatment of hypothyroidism, and three for nodule suppression). Nineteen developed new nodules while taking thyroxine. Three patients with treated primary hypothyroidism developed a carcinoma.
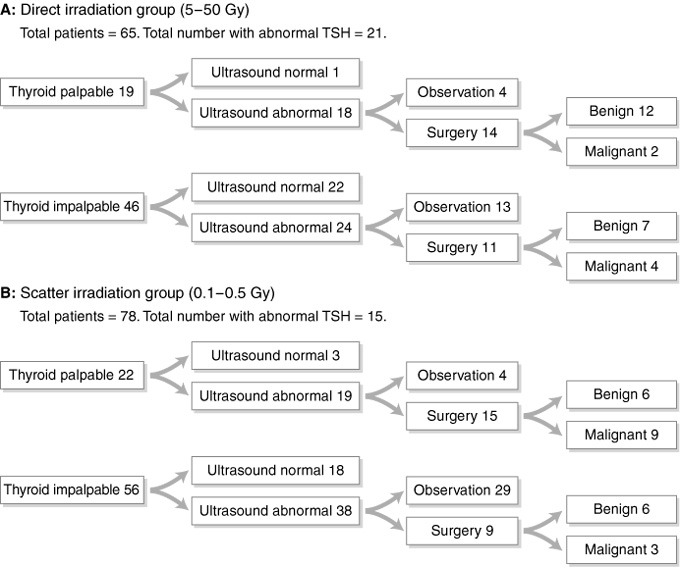
Box 2 shows a summary of the management outcomes for the two groups. Using combined palpation data and ultrasound data, the sensitivity of a positive ultrasound result was 70%, with specificity of 91%.
In the multivariate analysis, after adjusting for various prognostic factors, the only significant predictor of malignancy was having a palpable thyroid (OR, 4.1; 95% CI, 1.2–14; P = 0.03).
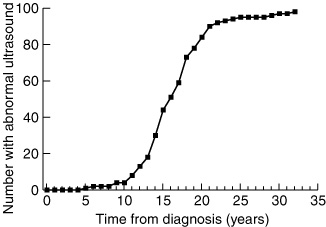
A peak in incidence of thyroid abnormality occurs from 10 to 25 years after original diagnosis (Box 3). The association is significant (OR, 1.2; 95% CI, 1.1–1.3; P < 0.01), indicating a 20% increase in the risk of an abnormal ultrasound result with each year after original diagnosis.
Radiation dose was not related to abnormal ultrasound result (P = 0.49), palpable thyroid (P = 1.0), or nodule status (P = 0.19).
Forty-nine subjects (34% of the study group; 38% and 31% for the direct and scatter groups, respectively) proceeded to surgery (Box 2). Forty-six subjects had a total thyroidectomy. Three subjects had a hemithyroidectomy (one of whom later developed nodules in the remaining lobe) performed by other surgeons.
The only important perioperative complication was transient hypocalcaemia in three patients. No subject had clinical lymph node enlargement at presentation. However, at surgery, two subjects had lymph node involvement and proceeded to a modified lymph node dissection.
Six subjects had papillary microcarcinoma (ie, a carcinoma < 1.0 cm).12 Of the remaining subjects, five showed unifocal papillary carcinoma (all in the scatter group), three showed multifocal papillary carcinoma, and four showed mixed papillary/follicular carcinoma.
The first important finding of our study is the appearance of cancer in thyroid glands exposed only to scatter irradiation in childhood. Our findings are likely to underestimate overall risk given that the risk of thyroid cancer is a lifetime risk,13 that the subjects reported here are still young, and that 50 subjects with abnormal ultrasound findings remain under observation.
In a similar clinical group, nodules were detected by ultrasound in a quarter of the subjects,14 significantly lower than in our study group (70%). Ten subjects (10%) had a surgical intervention and one of these had a papillary carcinoma, both figures significantly lower than in our study. In another study of the appearance of second cancers in childhood survivors of malignancy, thyroid cancer comprised 7.5% of the secondary cancers and, although radiation did not explain all of these, a large proportion of patients in the prophylactic cranial irradiation group was noted.15 Incidental nodular disease of the thyroid is uncommon in child population studies, with a prevalence of less than 3%.16
Although having a palpable thyroid was the only significant predictor of malignancy on multivariate analysis, seven patients with an impalpable thyroid (and abnormal thyroid ultrasound results) were subsequently found to have thyroid carcinoma. Thus, palpation should always be accompanied by thyroid ultrasound examination.
It is possible that radiation-induced thyroid neoplasia in childhood survivors of cancer is different from neoplasia arising spontaneously. Post-irradiation cancers arise in glands with widespread histological abnormality (Box 4), as in our study and as seen in children who were exposed to radiation in the Chernobyl disaster.17 Thyroid carcinoma in children and adolescents under standard conditions has an incidence of 0.5 per million per year.18 It is also unknown whether microcarcinomas in the young thyroid previously exposed to irradiation (present in a third of our patients) have different prognosis to those in older glands, where the long term prognosis is generally favourable.19
Our study raises issues concerning both immediate management and long term surveillance of people exposed to childhood irradiation of the thyroid. Our current guidelines for management of these patients are summarised in Box 5. A further consideration is that, although therapeutic ablative doses of radioactive iodine have not produced permanent gonadal damage in young patients with spontaneous thyroid cancer,21 a gonad already exposed to chemotherapeutic damage may be at high risk of sustaining damage from 131I.
The late effects of cancer therapy are with survivors for a lifetime. Survivors of childhood cancer need to be aware of the possibility of late effects, and develop some responsibility for their own long term surveillance. Doctors need to be aware that a past history of childhood cancer does not simply represent a cure, but also future health risks.
1: Original diagnoses for children in the direct and scatter irradiation groups
Diagnosis |
Direct
thyroid
irradiation |
Scatter
irradiation |
|||||||||
Acute lymphoblastic leukaemia/non-Hodgkin's lymphoma |
16 |
71 |
|||||||||
Hodgkin's disease |
13 |
0 |
|||||||||
Rhabdomyosarcoma |
10 |
1 |
|||||||||
Medulloblastoma |
8 |
0 |
|||||||||
Neuroblastoma |
5 |
1 |
|||||||||
Wilms' tumour |
5 |
0 |
|||||||||
Central nervous system tumour |
3 |
0 |
|||||||||
Histiocytosis |
2 |
0 |
|||||||||
Ewing's sarcoma |
1 |
0 |
|||||||||
Nasopharyngeal carcinoma |
1 |
0 |
|||||||||
Retinoblastoma |
1 |
0 |
|||||||||
Acute myeloid leukaemia |
0 |
3 |
|||||||||
Fanconi's anaemia |
0 |
1 |
|||||||||
2: Summary of management outcomes in patients exposed to direct (A) and scatter (B) irradiation
Six patients with malignant tumours (two in each of the direct thyroid palpable, direct thyroid impalpable and scatter thyroid palpable groups) had microcarcinoma. TSH = thyroid stimulating hormone.
4: Irradiated thyroid gland
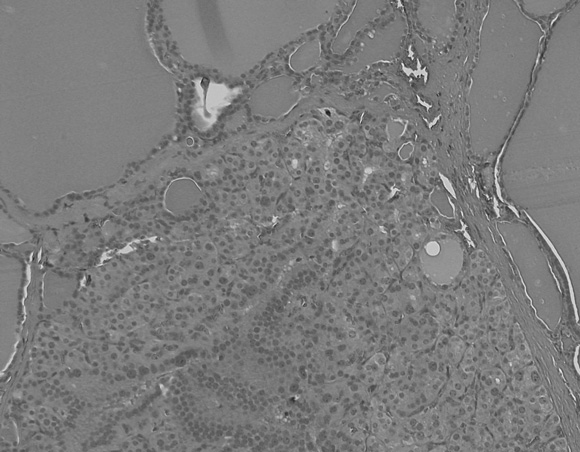
An irradiated thyroid gland containing a hypercellular nodule consisting almost entirely of microfollicles and showing focal nuclear atypia.
5: Guidelines for management of patients at risk of radiation-induced thyroid disease
At the completion of radiation treatment, inform the patient of the risk of future thyroid abnormality, including carcinoma, and emphasise the need for regular annual review.
Provide the patient with an information sheet that states:
– Palpation and thyroid function tests should be done, but are insufficient for detecting thyroid abnormality. Thyroglobulin levels may have a role in monitoring.
– Ultrasound is the most reliable test for detecting thyroid abnormality, and must form part of the annual review.
– Normal thyroid function tests do not exclude neoplasia.
Discuss total thyroidectomy as a reasonable option. The alternative is serial fine needle aspiration biopsy (FNAB). The presence of multiple nodules, the uncertainty as to whether the FNAB sample is representative (false negatives), repeated patient discomfort and a cancer patient's innate anxiety at the appearance of a nodule might make total thyroidectomy the preferred option. This procedure should be performed by an experienced head and neck surgeon, to avoid complications.
Counsel that thyroxine suppression does not protect against nodule formation or cancer.20
Received 24 August 2001, accepted 4 April 2002
- Helen M Somerville1
- Albert H Lam2
- Michael M Stevens3
- Kate S Steinbeck4
- Graham Stevens5
- Leigh W Delbridge6
- 1 The Children's Hospital at Westmead, Sydney, NSW.
- 2 Royal Prince Alfred Hospital, Sydney, NSW.
- 3 Royal North Shore Hospital, Sydney, NSW.
We thank I Firth for the diagram in Box 3, L Pearson and N Stuart for assistance with data management, J Rogers for secretarial assistance, and J Phillips for the picture in Box 4.
None declared.
- 1. Bleyer WA. The impact of childhood cancer on the United States and the world. CA Cancer J Clin 1990; 40: 355-367.
- 2. Soberman N, Leonidas JC, Cherrick I, et al. Sonographic abnormalities of the thyroid gland in long term survivors of Hodgkin's disease. Pediatr Radiol 1991; 21: 250-253.
- 3. Stewart RR, David CL, Eftekhari F, et al. Thyroid gland: US in patients with Hodgkin disease treated with radiation therapy in childhood. Radiology 1989; 172: 159-163.
- 4. Viswananthan K, Gierlowski TC, Scheider AB. Childhood thyroid cancer. Characteristics and long term outcome in children irradiated for benign conditions of the head and neck. Arch Pediatr Adolesc Med 1994; 148: 260-265.
- 5. Schneider AB, Shore-Freedman E, Ryo UY, et al. Radiation induced tumours of the head and neck following childhood irradiation. Prospective studies. Medicine (Baltimore) 1985; 64: 1-15.
- 6. Pacini F, Vorontsova T, Demidchuk EP, et al. Post-Chernobyl thyroid carcinoma in Belarus children and adolescents. Comparison with naturally occurring thyroid carcinoma in Italy and France. J Clin Endocrinol Metab 1997; 82: 3563-3569.
- 7. Kumpusalo L, Kumpasalo E, Soimakallio S, et al. Thyroid ultrasound findings 7 years after the Chernobyl accident. A comparative epidemiological study in the Bryansk region of Russia. Acta Radiol 1996; 37: 904-909.
- 8. Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res 1995; 141: 259-277.
- 9. Stevens G, Downes S, Ralston A. Thyroid dose in children undergoing prophylactic cranial irradiation. Int J Radiat Oncol Biol Phys 1998; 42: 385-390.
- 10. Microsoft Excel [computer program]. Version 9 (with Ecstat extension version 1.8C). Redmond: Microsoft, 2002.
- 11. SPSS for Windows [computer program]. Version 11. Cary, North Carolina: SAS Institute, 2002.
- 12. Rosai J. Tumors of the thyroid gland. Atlas of tumor pathology. Washington DC: Armed Forces Institute of Pathology, 1992.
- 13. Same DS, Schneider AB. External radiation and thyroid neoplasia. Endocrinol Metab Clin North Am 1996; 25: 181-195.
- 14. Crom DB, Kaste SC, Tubergen DG, et al. Ultrasonography for thyroid screening after head and neck irradiation in childhood cancer survivors. Med Pediatr Oncol 1997; 28: 15-21.
- 15. Black P, Straaten A, Gutjahr P. Secondary thyroid carcinoma after treatment for childhood cancer. Med Pediatr Oncol 1998; 31: 91-95.
- 16. Hampel R, Kulberg T, Klein K, et al. Goiter incidence in Germany is greater than previously expected. Med Klin 1995; 90: 324-329 (In German.)
- 17. Nikifurov Y, Heffess CS, Korzenko AV, et al. Characteristics of follicular tumours and nonneoplastic thyroid lesions in children and adolescents exposed to radiation as a result of the Chernobyl disaster. Cancer 1995; 76: 900-909.
- 18. Danese D, Gardini A, Farsetti A, et al. Thyroid carcinoma in children and adolescents. Eur J Pediatr 1997; 156: 190-194.
- 19. Baudin E, Travagli JP, Ropers J, et al. Microcarcinoma of the thyroid gland: the Gustave-Roussy Institute experience. Cancer 1998; 83: 553-559.
- 20. Shafford EA, Kingston JE, Healy JC, et al. Thyroid nodular disease after radiotherapy to the neck for childhood Hodgkin's disease. Br J Cancer 1999; 80: 808-814.
- 21. Casara D, Rubello D, Saladini G, et al. Pregnancy after high therapeutic doses of iodine-131 in differentiated thyroid cancer: potential risks and recommendations. Eur J Nucl Med 1993; 20: 192-194.





Abstract
Objectives: To describe a cohort of survivors of childhood malignancy at risk of developing thyroid abnormality, and propose guidelines for management of such patients.
Design: Retrospective case series.
Setting: Late-effects oncology clinic at a large children's hospital in Sydney.
Subjects: 142 patients who had received irradiation to the thyroid from the 1970s onwards, who attended the late-effects clinic from May 1989 to December 1998.
Interventions: Thyroid palpation by an endocrinologist or surgeon, serum thyroid-stimulating hormone assay and thyroid ultrasound examination were performed on all subjects and, depending on findings, some subjects proceeded to fine-needle biopsy or surgery (total thyroidectomy). A few patients required adjuvant 131I administration.
Outcome measures: Radiation dose received; results of thyroid palpation; thyroid function tests; ultrasound findings; diagnosis of the abnormalities; and outcomes of surgical interventions.
Results: 49 subjects (24 of 65 patients who received scatter irradiation to the thyroid and 25 of 78 patients who received direct irradiation) had thyroid surgery. Of these, 12 in the scatter and six in the direct irradiation group were found to have thyroid malignancy. Fifty subjects with abnormal ultrasound results remain under surveillance. Having a palpable thyroid was predictive of malignancy, but age at original diagnosis, sex, current age, time since irradiation, radiation dose, nodule type and nodal involvement were not.
Conclusion: There is a significant risk of cancer in thyroid glands exposed to radiation as part of therapy for childhood cancer. This risk is greater for patients who received scatter (versus direct) irradiation. Nodular change is usually not apparent for many years, so lifelong surveillance is necessary. Palpation alone is not sufficient to detect thyroid cancer and thyroid ultrasound examination is recommended.