Although many of these infections are trivial, some may threaten life or limb
Soft tissue infections can be subdivided according to the skin compartment involved (Box 1). Diagnosis is based on the appearance of lesions, degree of pain and systemic toxicity. Knowledge of the organisms involved does not always help define the tissue depth of disease, but aids choice of antimicrobial therapy. Investigation and management of common soft tissue infections are summarised in Box 1.
Infection of the hair follicles may occur after exfoliation, use of a loofah sponge and shaving, or may be spontaneous. A specific form is "whirlpool" ("hot tub" or "spa") folliculitis, caused by Pseudomonas aeruginosa.5
S. aureus is the leading pathogen that causes furuncles and abscesses in the community. Certain phage types are associated with recurrent episodes of furunculosis and may spread among family members. Staphylococcal bacteraemia may result from a minor skin lesion or furuncle, with potentially severe complications, including osteomyelitis, septic arthritis and endocarditis.6
Recently, infections caused by community-acquired, methicillin-resistant S. aureus have been described in both adults and children in Australia.7 These bacteria are resistant to β-lactam antibiotics, but retain susceptibility to agents such as clindamycin, in contrast to multiresistant S. aureus, which is usually hospital-acquired. This β-lactam resistance may delay effective therapy (case history, Box 3).
In patients with recurrent S. aureus soft tissue infection, attempts can be made to eradicate the causative strain from long-term carriage in the nasal passages and on skin with nasal mupirocin and skin antiseptic solutions.8 Penicillins and cephalosporins are ineffective in eradicating nasal carriage.
Cellulitis is an acute, spreading inflammation involving the epidermis, dermis and subcutaneous fat. The most common causes are S. aureus and ß-haemolytic streptococci (most commonly group A [S. pyogenes], but also groups C and G). Host factors may predispose to cellulitis and should be corrected if possible. These are infective (eg, varicella, tinea pedis and scabies) and non-infective (eg, eczema, trauma, local ischaemia, venous, arterial or vasculitic ulceration, lymphatic impairment, surgery and radiotherapy). Rarer causes of cellulitis are associated with specific exposures (Box 4). Infection with S. pyogenes may also be confined to the dermis, termed erysipelas.
In practice, the specific causative organism is usually not isolated: blood cultures are usually negative,10 and skin swabs are rarely diagnostic. However, culture of an aspirate from an intact blister may be helpful, and, in the immunosuppressed patient or after unusual exposures (Box 4), an aspirate obtained through needling the leading edge of the cellulitis is valuable.11
Intravenous antibiotics are preferred initially except in mild infections (Box 1). Features that suggest a poor prognosis and need for hospital admission are shown in Box 5. Home-based intravenous therapy is an alternative for patients in a stable condition.12
If cellulitis does not respond to empirical β-lactam therapy, then environmental or occupational exposure to unusual pathogens should be considered (Box 4). Infections with these organisms may also present as single ulcers, nodules or nodular lymphangitis (case history, Box 6). Tissue biopsy may be required for diagnosis (Box 1).
Some patients have recurrent cellulitis and can be provided with a supply of antibiotics to take when symptoms recur. However, this is not always effective. Twice-daily penicillin V, cephalexin or erythromycin may be effective prophylaxis,13 while some patients require monthly injections of benzathine penicillin.
Necrotising fasciitis is a surgical emergency needing prompt recognition and radical debridement of devitalised tissue. It can take two forms:
type 1, caused by multiple organisms, often of gut origin (synergistic gangrene); and
type 2, caused by group A streptococci.
Both forms cause severe systemic toxicity that leads to hypotension, respiratory distress and multiorgan failure. There is usually severe pain in the affected area, with only modest overlying skin changes in the early stages, progressing rapidly to fascial and skin necrosis (Box 7) and deep tissue infarction, particularly of the muscle layers.
Types of bone and joint infections and their investigation and management are summarised in Box 8.
Septic arthritis requires prompt diagnosis, as delays in surgical drainage and antibiotic therapy may lead to progressive synovitis and irreversible cartilage and bone destruction.14-16
This relies on a combination of clinical assessment, laboratory and radiological investigations. Attention should be paid to:
distribution of involved joints;
pre-existing joint disease, trauma or extra-articular infection;
underlying diseases (eg, immunosuppression, malignancy or diabetes mellitus);
sexual history (eg, recent genital tract discharge or urethritis); and
activities and travel history (infections such as melioidosis and brucellosis have strong geographic associations).
Blood leukocytosis and raised C-reactive protein level and erythrocyte sedimentation rate are characteristic. Blood cultures are positive in about 50% of patients with acute non-gonococcal bacterial arthritis but in only 20% of those with gonococcal arthritis.17 Serological tests are rarely helpful, except in chronic infections with Brucella spp., Coxiella burnetii (Q fever), Treponema pallidum (syphilis) and Borrelia burgdorferi (Lyme disease).
Culture and microscopy of synovial fluid are essential for specific diagnosis of a hot, swollen joint. Gram stain shows organisms in 50% of cases of septic arthritis. Ultrasonography is very sensitive for detecting joint effusions and guiding diagnostic aspiration, especially in the hip joint. Computed tomography and magnetic resonance imaging are useful in complicated or atypical cases.18
Infection of prosthetic joints usually presents with insidious onset of increasing joint pain, modest swelling and possibly sinus formation.19 Presentation may be more acute if infection is haematogenous or occurs early after placement of a prosthesis.
Plain x-rays may show loosening of the prosthesis, but no clinical or radiological features can reliably differentiate infective from non-infective loosening. Radionuclide scans may show increased uptake at the site of infection but cannot provide a specific diagnosis. Blood cultures are rarely positive, except in acute infections, and microscopy and culture of swabs from sinuses are usually not helpful. However, specimens obtained from joint aspirates or deep debrided tissue may show the causative organism.20,21
Prosthesis salvage can be attempted when the prosthesis is not loose and onset of symptoms is acute. With this approach, infections due to S. aureus have a greater than 50% chance of cure if surgical debridement is performed early.22,23 Broader application of this approach has been shown to be cost-effective in the elderly.24 If salvage is not realistic, the infected prosthesis is removed or exchanged. A two-stage procedure is widely used, with an interval of about six weeks of antibiotic therapy before replacement of the prosthesis. If two operative procedures are not feasible, exchange of the prosthesis in one operation may achieve reasonable results.
Bone becomes infected either through haematogenous spread of organisms, or, particularly in people with peripheral vascular disease, secondary to a contiguous focus of infection.25 Osteomyelitis can be either acute or chronic. The latter usually reflects the presence of non-viable bone or other material and is characterised by bone loss, persistent drainage from sinus tracts and sequestra. The natural history often includes relapses and remissions.
Intravenous antibiotic therapy is required in all cases, at least initially (Box 8). Attempts should always be made to identify the causative organism, as selection of appropriate antibiotics is important for a good outcome.26 Chronic osteomyelitis requires surgical debridement to remove devitalised bone and soft tissue for cure.
About 15% of people with diabetes mellitus develop foot ulceration,27 which is complicated by osteomyelitis in two-thirds of cases.28 Foot infection is a leading cause of hospital admission in people with diabetes and a major cause of lower-extremity amputation. Factors that increase the risk of osteomyelitis are:
duration of diabetes mellitus over 10 years;
peripheral neuropathy;
abnormal foot structure with maldistribution of weight over the plantar surface of the foot;
peripheral vascular disease;
poor glycaemic control;
disruption of skin integrity (eg, penetrating injury, fungal infection); and
male sex.
An approach to assessment and management of suspected diabetic foot infection and osteomyelitis, as well as practical tips, are summarised in Box 9. Infection complicating foot ulceration is suggested by spreading redness around the ulcer, local swelling and systemic features (eg, malaise, fever and night sweats). Assessing the depth of infection beneath the ulcer and differentiating infection from neuropathic change is particularly difficult, as both cause bone destruction.
Imaging for osteomyelitis of the foot has poor specificity in diabetic patients, as in non-diabetic patients. In addition, neuropathic osteoarthropathy and healing fractures in diabetic patients may further reduce specificity.30 Culture of superficial swabs is not useful, as diabetic foot ulcers are invariably colonised by multiple organisms. Bone biopsy and culture is the definitive investigation, with an estimated sensitivity of 95% and specificity of 99%,31 but its value is much reduced by concurrent antibiotic therapy.
Patients with diabetes should be educated about avoiding skin trauma; wearing protective, well-fitting footwear; skin moisturising; and inspecting daily for skin pressure. Regular review by a podiatrist is important.
Established osteomyelitis is difficult to cure in the presence of diabetic arteriopathy. Relapse is common, and suppression is often the best that can be achieved. Surgical revascularisation is feasible in some patients and leads to better short- and long-term outcomes. Antimicrobial therapy should be begun pending results of deep tissue culture and is required for at least two weeks for soft tissue infection, and for at least six weeks (often considerably longer) for osteomyelitis32 (Box 10). Surgical resection of infected bone is the most effective means to eradicate infection, with maintenance of functional integrity of the foot as the goal.
With aggressive, targeted antibiotic therapy and early debridement, the short-term outcome is generally good. However, the three-year recurrence rate is high (amputation in more than 22% of patients), as is three-year mortality (27% in patients with primary healing of the initial ulcer, and 41% in those requiring amputation as initial treatment).33
Evidence-based recommendations
Septic arthritis can be diagnosed on the basis of the clinical picture, supported by results of microscopy and culture of joint fluid17 (E3).
In prosthetic joint infection, the most consistent results rest with prosthesis removal and antibiotic therapy before replacement; long term success depends on many factors,23,34 and it may not be practical for some patients. In selected patients, retention of an infected prosthetic hip arthroplasty plus prolonged antibiotic therapy may be successful (E2).
Osteomyelitis is suggested in diabetic foot infection if the overlying ulceration is deep, or bone can be reached by probing27,29 (E2).
Resection of devitalised bone and tissue plus antibiotic therapy can be an effective foot-sparing approach in diabetic foot osteomyelitis35 (E2).
1: Investigation and management of soft tissue infections*
Syndrome |
Common pathogens |
Investigations |
Management |
||||||||
Impetigo (epidermis) |
Streptococcus pyogenes Staphylococcus aureus |
|
|||||||||
Folliculitis (hair follicle) |
S. aureus Pseudomonas aeruginosa ("spa" or "whirlpool" folliculitis) |
|
|
||||||||
Skin abscesses, furuncles (hair follicles) |
S. aureus |
|
|
||||||||
Cellulitis (epidermis, dermis, subcutaneous fat) |
ß-Haemolytic
streptococci |
|
|
||||||||
Necrotising fasciitis (fascia) |
S. pyogenes
|
|
|
||||||||
Clostridial myonecrosis (gas gangrene) (muscle) |
Clostridium perfringens |
|
|
||||||||
3: Case history — community-acquired methicillin-resistant Staphylococcus aureus
Presentation: A 14-year-old girl presented with a four-week history of progressive swelling in the left submandibular region. Despite treatment with oral flucloxacillin for 10 days she experienced increasing pain, fever and torticollis.
Management: The girl was admitted to hospital and given intravenous flucloxacillin and penicillin for three days without improvement.
Further history revealed that both the girl and her younger brother had experienced recurrent boils since a family visit to Samoa a year previously. Swabs of pus from a boil, as well as from her nose, axilla and groin, had failed to show any pathogens. The boils had responded slowly to flucloxacillin. Attempted eradication of staphylococci with intranasal mupirocin and triclosan washes had prevented further skin sepsis.
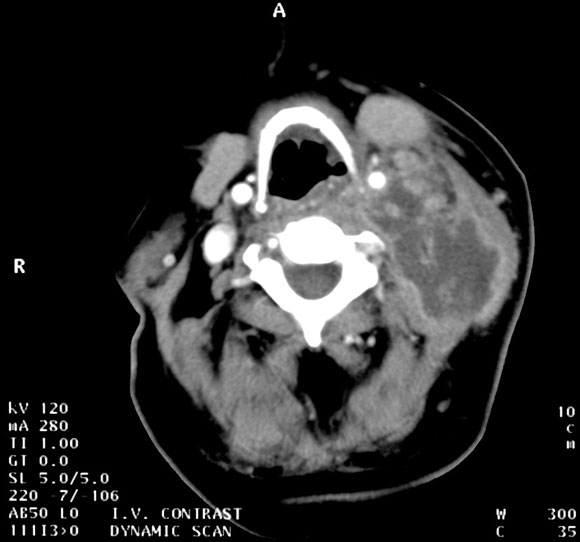
Investigations: Magnetic resonance imaging of the neck demonstrated necrotic submandibular lymphadenitis with a deep cervical abscess (right). The abscess was drained under anaesthesia. Culture of the pus revealed "community type" or "non-multiresistant" methicillin-resistant Staphylococcus aureus. The isolate was susceptible to erythromycin, tetracycline, gentamicin and ciprofloxacin, and resistant to β-lactams, flucloxacillin and cephalexin.
Management and course: Treatment was changed to clindamycin. Over the next 24 hours, the fever abated, and within seven days the swelling and pain decreased. Therapy was changed to oral clindamycin. After a further four weeks' therapy, the infection was completely resolved.
Methicillin-resistant S. aureus (MRSA) is no longer confined to patients with a history of recent hospitalisation.
"Community" MRSA strains are increasingly reported in children and are often associated with pyogenic complications.
Failure to consider or recognise MRSA may lead to inappropriate β-lactam therapy. We believe that boils and furuncles should be swabbed for Gram stain and culture.
4: Unusual causes of cellulitis and nodular infections associated with specific exposures
Traumatic inoculation of soil, penetrating injury from thorns: Non-tuberculous mycobacteria (eg, Mycobacterium fortuitum, M. chelonae, M. ulcerans), Nocardia spp., fungi
Travel to tropical Australia9 or other tropical areas: Burkholderia pseudomallei (melioidosis), chromoblastomycosis, Chromobacterium violaceum
Travel to tropical areas (beaches): Cutaneous larva migrans
Aquatic or marine trauma: Mycobacterium marinum (case history, Box 6), erysipelothrix (erysipeloid), Vibrio or Aeromonas infection
Animal bites: Pasteurella multocida, Capnocytophaga canimorsus
Human bites: Eikenella corrodens
Bathing in spas or tubs: Pseudomonas aeruginosa
Cat scratch: Bartonella henselae
Immunosuppression (eg, HIV, transplantation): Nocardia spp., Cryptococcus neoformans, Mycobacterium tuberculosis (reactivation)
5: Features suggesting a poor prognosis and need for hospital admission in cellulitis
Pre-existing conditions (eg, renal impairment, diabetes, congestive cardiac failure, peripheral vascular disease, neoplasm, radiotherapy in proximity to cellulitis, immunosuppression, splenectomy, alcoholism and neutropenia)
Extensive or rapidly progressive cellulitis
Presence of bullae, necrosis or muscle involvement
High fever, rigors
Hypotension (with or without generalised rash)
Development of renal impairment
Suppurative wound or bite (especially on face or hand) requiring surgical drainage
Animal or human bite; exposure to marine or riverine waters
Lack of systemic or local response, or rising or unchanging C-reactive protein level despite adequate therapy
Positive blood cultures
6: Case history — soft tissue infection caused by an unusual pathogen
Presentation: An elderly woman injured her hand with a fish hook while rock fishing on the New South Wales central coast. Over the next three weeks, she developed small, tender nodules along the lymphatic chain of the right forearm (pictured).
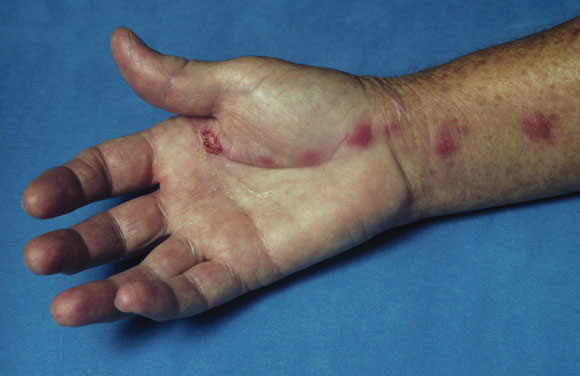
Investigation: A swab from a palmar ulcer grew Staphylococcus aureus. However, the illness had no systemic features (no fever, normal white cell count and no lymphadenopathy). She was assumed to have nodular lymphangitis. Biopsy of the lesion confirmed the presence of acid-fast bacilli. Mycobacterium marinum was isolated after three weeks' incubation.
Management: She was treated with trimethoprim–sulfamethoxazole for three months with complete resolution.
This case illustrates the importance of a good history and review of the clinical presentation; the illness was not compatible with a staphylococcal infection despite a positive culture result.
Post-traumatic infections caused by Mycobacterium or Nocardia spp. may persist for weeks or months; diagnosis may require biopsy.
Biopsy specimens should be sent for histopathological and microbiological examination, and the possibility of an unusual pathogen (eg, a waterborne pathogen) should be specified, as the laboratory may not culture on the specific media required for isolation of these organisms unless prompted.
7: Necrotising fasciitis
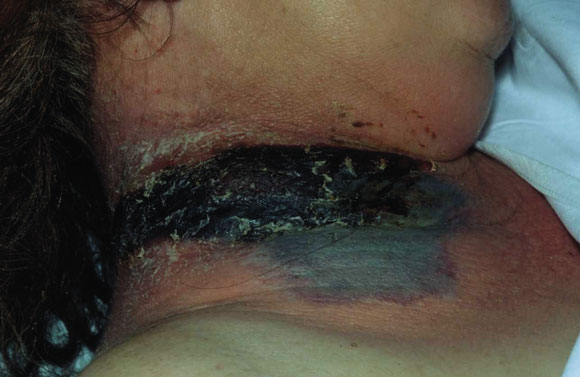
Necrosis involving skin and deep structures of the neck 48 hours after presentation with pain and fever.
8: Investigation and management of bone and joint infections
Syndrome |
Common pathogens |
Microbiological investigations |
Management |
||||||||
Native joint septic arthritis |
Staphylococcus aureus
|
|
|
||||||||
Prosthetic joint infection |
Coagulase-negative staphylococci
|
|
|
||||||||
Osteomyelitis |
S. aureus
|
|
|
||||||||
9: Assessment and management of suspected diabetic foot infection and osteomyelitis
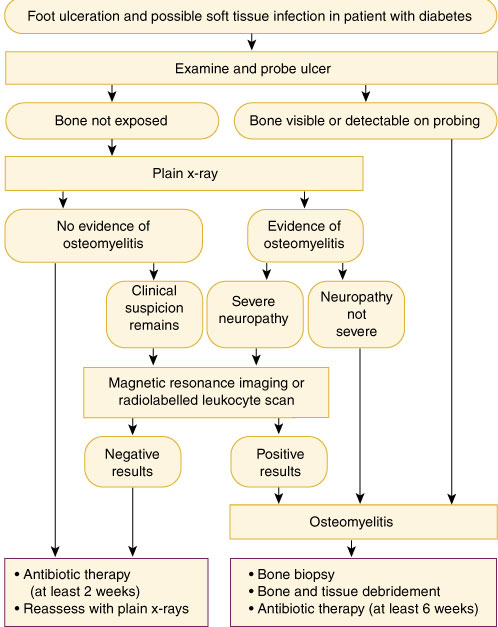
Practical tips for assessing a diabetic foot ulcer
Previous episodes of osteomyelitis or neuropathic complications increase the risk of further episodes of osteomyelitis.
Most diabetic patients with foot infection have no fever.
Ulceration and soft tissue infection present for over two weeks, particularly over bony prominences, have a high risk for bony involvement.
All ulcers with exposed bone have underlying osteomyelitis.
Tapping bone while probing an ulcer with a sterile blunt probe is a sensitive (66%) and specific (85%) bedside test for osteomyelitis. However, as the negative predictive value is only 56%, this test cannot exclude osteomyelitis.29
10: Empirical antibiotic therapy for diabetic foot infection
Mild to moderate soft tissue infection Metronidazole (400 mg orally, 12-hourly) plus cephalexin (500 mg orally, 6-hourly) or amoxycillin–clavulanate (875 mg/125 mg orally, 12-hourly)
Severe soft tissue infection or osteomyelitis Ticarcillin–clavulanate (3.1 g intravenously, 6–8 hourly) or clindamycin (600 mg intravenously, 8-hourly) plus ciprofloxacin (750 mg orally, 12-hourly)
- Thomas Gottlieb1
- Bridget L Atkins2
- David R Shaw3
- Series Editors:
- 1 Department of Microbiology and Infectious Diseases, Concord Hospital, Sydney, NSW.
- 2 Oxford Radcliffe Hospitals, Nuffield Orthopaedic Centre, Oxford, UK.
- 3 Infectious Diseases Unit, Royal Adelaide Hospital, Adelaide, SA.
- 1. Therapeutic Guidelines Limited. Therapeutic guidelines: antibiotic. Version 11, 2000. Melbourne: Therapeutic Guidelines Limited, 2000.
- 2. Koning S, van Suijlekom-Smit LW, Nouwen JL, et al. Fusidic acid cream in the treatment of impetigo in general practice: double blind randomised placebo controlled trial. BMJ 2002; 324: 203-206.
- 3. Clinical practice guidelines of the Royal Children's Hospital, Melbourne, Australia. Available at <http://www.rch.unimelb.edu.au/clinicalguide> Accessed May 2002.
- 4. Rogers M, Dorman DC, Gapes M, Ly J. A three-year study of impetigo in Sydney. Med J Aust 1987; 147: 63-65.
- 5. Gibson AR, De Jager J, McCrossin I. Pseudomonas folliculitis associated with the use of health-spa whirlpools. Med J Aust 1983; 1: 381-383.
- 6. O'Kane G, Gottlieb T, Bradbury R. Staphylococcal bacteraemia: the hospital or the home? A review of Staphylococcus aureus bacteraemia at Concord Hospital in 1993. Aust N Z J Med 1998; 28: 23-27.
- 7. Collignon P, Gosbell I, Vickery A, et al. Community-acquired methicillin-resistant Staphylococcus aureus in Australia. Lancet 1998; 352: 145-146.
- 8. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 1997; 10: 505-520.
- 9. Currie BJ, Carapetis JR. Skin infections and infestations in Aboriginal communities in northern Australia. Australas J Dermatol 2000; 41: 139-143.
- 10. Bisno AL, Stevens DL. Streptococcal infections of skin and soft tissues. N Engl J Med 1996; 334: 240-245.
- 11. Sachs MK. The optimum use of needle aspiration in the bacteriologic diagnosis for cellulitis in adults. Arch Intern Med 1990; 150: 1907-1912.
- 12. Leder K, Turnidge JD, Grayson ML. Home based treatment of cellulitis with twice-daily cefazolin. Med J Aust 1998; 169: 519-522.
- 13. Kremer M, Zuckerman R, Avraham Z, et al. Long term antibiotic therapy in the prevention of recurrent soft tissue infections. J Infect 1991; 22: 37-40.
- 14. Esterhai JL, Gelb I. Adult septic arthritis. Orthop Clin North Am 1991; 22: 503-514.
- 15. Morgan DS, Fisher D, Merianos A, Currie BJ. An 18 year experience of septic arthritis from tropical Australia. Epidemiol Infect 1996; 117: 423-428.
- 16. Youssef PP, York JR. Septic arthritis: a second decade of experience. Aust N Z J Med 1994; 24: 307-311.
- 17. Atkins BL, Bowler IEB. The diagnosis of large joint sepsis. A review. J Hospital Infect 1998; 40: 263-274.
- 18. Brower AC. Septic arthritis. Radiol Clin North Am 1996; 34: 293-230.
- 19. Steckelberg JM, Osmon DR. Prosthetic joint infections. In: Bisno AL, Waldvogel FA, editors. Infections associated with indwelling medical devices. 2nd ed. Washington DC: ASM Press, 1994: 259-290.
- 20. Atkins BL, Athanasou N, Deeks JJ, et al, and the OSIRIS study group. Prospective evaluation of criteria for microbiological diagnosis of prosthetic joint infection at revision arthroplasty. J Clin Microbiol 1998; 36: 2932-2939.
- 21. Atkins BL, Berendt AR. Prosthetic joint infection. In: Bulstrode C, Buckwalter J, Carr A, et al, editors. Oxford textbook of orthopaedics and trauma. Oxford: Oxford University Press, 2002: 1443-1454.
- 22. Brandt CM, Sistrunk WW, Duffy MC, et al. Staphylococcus aureus prosthetic joint infection treated with debridement and prosthesis retention. Clin Infect Dis 1997; 24: 914-919.
- 23. Zimmerli W, Widmer AF, Blatter M, et al. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA 1998; 279: 1537-1541.
- 24. Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis 2001; 32: 419-430.
- 25. Mader J, Shirtliff M, Calhoun JH. Staging and staging application in osteomyelitis. Clin Infect Dis 1997; 25: 1303-1309.
- 26. Lew DP, Waldvogel FA. Osteomyelitis. N Engl J Med 1997; 336: 999-1007.
- 27. Lipsky BA. Osteomyelitis of the foot in diabetic patients. Clin Infect Dis 1997; 25: 1318-1326.
- 28. Caputo GM, Cavanagh PR, Ulbrecht JS, et al. Assessment and management of foot disease in patients with diabetes. N Engl J Med 1994; 331: 854-860.
- 29. Grayson, ML, Gibbons GW, Galogh K, et al. Probing to bone in infected pedal ulcers. JAMA 1995; 9: 721-723.
- 30. Tomas MB, Patel M, Marwin SE, Palestro CJ. The diabetic foot. Br J Radiol 2000; 73: 443-450.
- 31. Mushlin A, Littenburg B. Diagnosing pedal osteomyelitis: testing choices and their consequences. J Gen Intern Med 1994; 9: 1-7.
- 32. Grayson ML. Diabetic foot infections. Antimicrobial therapy. Infect Dis Clin North Am 1995; 9: 143-161.
- 33. Apelqvist J, Larrson J, Agardh CD. Long-term prognosis for diabetic patients with foot ulcers. J Intern Med 1993; 233: 485-491.
- 34. Widmer AF. New developments in diagnosis and treatment of infection in orthopedic implants. Clin Infect Dis 2001; 33 Suppl 2: S94-S106.
- 35. Karchmer AW, Gibbons GW. Foot infections in diabetes: evaluation and management. Curr Clin Top Infect Dis 1994; 14: 1-22.





Abstract
Soft tissue infections are common and usually respond rapidly to oral antibiotics; if empirical therapy fails then exposure to unusual organisms should be considered.
Septic arthritis requires early recognition, identification of the infecting pathogen and urgent joint washout to prevent irreversible cartilage and bone destruction.
Prosthetic joint infection is uncommon but has high morbidity; the best outcomes are achieved with removal of the prosthesis and replacement after at least six weeks of antibiotic therapy.
Osteomyelitis often complicates diabetic foot infection with ulceration and is rarely cured by antibiotics alone; early surgical intervention achieves the best outcome.