Sexually transmitted infections (STIs) remain endemic in Australia and have significant individual and public health consequences.
Common presentations of STIs and the recommended investigations are summarised in Box 1. However, most STIs are asymptomatic or produce mild symptoms that do not bring affected people to medical attention. These STIs will be detected only through screening and contact tracing. Patients with a suspected or diagnosed STI should be screened for other STIs, with the tests performed dependent on the probability of specific infections in the individual. Partners of those diagnosed with an STI should be screened and treated empirically.
Infection with Chlamydia trachomatis is often asymptomatic and causes cervicitis, endometritis and pelvic inflammatory disease in women, with the sequelae of tubal factor infertility and ectopic pregnancy. In men, it causes urethritis, epididymo-orchitis and prostatitis (Box 2). Infection with C. trachomatis is the third most common notifiable disease in Australia, with 16 770 cases reported in 2000 (Communicable Diseases System, personal communication1). As most infections in women are asymptomatic, notification data may under-represent the true incidence of disease in the community. There are no agreed national screening guidelines for C. trachomatis infection, but candidates for screening include sexually active young women attending for Pap smears, contraceptive advice, antenatal care or termination of pregnancy, if indicated by their sexual history (case history, Box 3).
Nucleic acid amplification techniques have largely replaced tissue culture, non-amplification DNA probe assays and other rapid antigen assays (eg, direct immunofluorescence and enzyme immunoassay) as the "gold standard" for diagnosis of C. trachomatis because of their greater sensitivity.2 Nucleic acid amplification techniques may use samples collected from the cervix and vagina, but are also easily adapted to self-sampling with self-inserted swabs, tampons or first-void urine.3,4 Most laboratories use PCR, ligase chain reaction or strand displacement amplification. These techniques can be performed in less than 24 hours, but most tests are "batched" and performed several times a week.
A major advance in treatment of C. trachomatis infection has been the introduction of azithromycin (Box 4); clinical trials have found that a single oral dose of this drug has equivalent efficacy to a seven-day course of twice-daily oral doxycycline when compliance is high.5 Both drugs achieve cure rates greater than 95%. If compliance is likely to be suboptimal, then azithromycin is more cost effective.6 Azithromycin is also effective for treatment of non-specific urethritis (ie, cases with negative results for chlamydia and gonococcus).7 Its risk in pregnancy is categorised as B1, and it can be used safely during pregnancy and breastfeeding.8 A seven-day course of erythromycin, roxithromycin or amoxycillin has been recommended for use in pregnancy, but, as these agents are less effective, careful follow-up and retesting after three weeks is highly recommended.9
Complicated chlamydial infections, such as epididymitis or pelvic inflammatory disease (PID), require longer therapy, but treatment recommendations vary greatly. For PID in general, broad-spectrum antimicrobials have been advised (Box 4), as the infection usually involves mixed endogenous vaginal flora, particularly if it occurs postpartum or after gynaecological instrumentation, miscarriage, termination of pregnancy, or insertion of an intrauterine contraceptive device. If PID is sexually acquired, an antibiotic to cover Neiserria gonorrhoeae is also required. As the definitive diagnosis of PID requires laparoscopy, and as the severity and number of episodes of untreated disease contribute to the risk of subsequent complications (chronic pelvic pain, infertility and ectopic pregnancy),10 therapy must be based on clinical features.
Gonorrhoea has similar symptoms, signs and sequelae to chlamydial infection, although symptomatic disease may be more common, especially in men. Rates of gonorrhoea in Australia are currently at their highest for nearly 20 years and double those of 10 years ago.11 Three groups are primarily affected: men who have sex with men (the group with the greatest increase), Indigenous Australians in remote settings, and people who have heterosexual contact overseas. Other heterosexual cases are uncommon.11
Culture of Neisseria gonorrhoeae from a urethral or endocervical swab remains the standard diagnostic method, as information on antibiotic susceptibility is essential. However nucleic acid amplification of urine, cervical and vaginal specimens has an important role in diagnosis of this fastidious organism, especially where prevalence is high and where there may be delay between specimen collection and processing. A PCR assay for both C. trachomatis and N. gonorrhoeae is available in a single tube. Because specificity is lower for N. gonorrhoeae than for C. trachomatis,12 it is recommended that all positive results for N. gonorrhoeae should be confirmed by amplification of an alternative target (such as the 16S rRNA gene) or preferably by culture. A ligase chain reaction test for N. gonorrheae is also available. Testing for both organisms is currently available through the Medicare schedule.
Treatment is summarised in Box 4. Infections acquired in remote Australia are almost universally penicillin-sensitive (although the minimum inhibitory concentration for new isolates has been slowly rising over time), and oral amoxycillin with probenecid can be used. Infections acquired in other parts of Australia are often penicillin-resistant,13 and intramuscular ceftriaxone or the oral quinolone ciprofloxacin should be used. However, there has been a recent increase in the incidence of quinolone resistance in isolates obtained from homosexually active men in New South Wales,13 and from heterosexual people infected in South East Asia. Where local data indicate that quinolone resistance is rare, ciprofloxacin can be used for empirical therapy; otherwise, intramuscular ceftriaxone should be used in the first instance. When treating gonococcal infection, antibiotic therapy should also cover possible chlamydial co-infection.
Penicillin alone is not sufficient for pharyngeal and anorectal infections, even with penicillin-sensitive strains. Therefore, in homosexually active men, ceftriaxone or ciprofloxacin is required. Urethral and anorectal infection will be cured in over 98% of cases, whereas for pharyngeal infection the cure rate is lower (about 90%).9
Infection with the protozoan Trichomonas vaginalis is the most common treatable STI in the world. Prevalence has fallen progressively in the developed world over the past 20 years, but in women living in remote areas of Australia may be as high as 25%.14
T. vaginalis may be diagnosed by Pap smear (which has poor sensitivity and specificity), culture or microscopy of a wet preparation. Enzyme immunoassay (EIA) and immunofluorescence tests are available but not widely used. In-house nucleic acid amplification methods are used by some Australian laboratories and have high sensitivity and specificity15 compared with the "gold standard" of culture. They have been used to detect the organism in urine, self-inserted swabs and self-collected tampon samples and are suitable where access to medical personnel is limited.
Trichomonal infection is treated with a single dose of metronidazole or tinidazole (Box 4). Most studies of treatment efficacy have been conducted in women, and there is a surprising lack of evidence regarding their efficacy in men.
Trichomonal infection in pregnancy is associated with premature rupture of membranes and premature labour. However, a recent study showed that treatment of asymptomatic infection during pregnancy is also associated with an increased rate of premature labour.16 This highlights the need to prevent and treat infections in women of childbearing age before pregnancy.
Infection with one of the 30 human papillomavirus (HPV) types that infect the genital epithelium is very common, although the vast majority of these infections are asymptomatic.17 A study of women attending a United States university found that the proportion with HPV detected by PCR was 30% in the first year after initiation of sexual intercourse and 80% after 56 months.18 The most common virus type was HPV 16, followed by 6 and 11.
Infection with "oncogenic" strains of HPV has been identified as one of the main risk factors for developing cervical and other anogenital cancers.19 HPV types 16 and 18 account for about 80% of the "high-risk" types, with the remainder being 31, 33, 35, 45, 51, 52, 58 and 59.19
Diagnosis of external genital warts is almost always clinical. They can be treated by several practitioner-applied therapies (Box 5), which produce similar initial responses (50% to 70%), but relapse may be less common with the more expensive imiquimod (Box 6).18 This new immune-response modifier has a good safety profile and high efficacy and can also be self-administered. Only cryotherapy is definitely safe in pregnancy. Condoms reduce but do not eliminate the risk of HPV transmission.
Vaccines for HPV are in development, but their role in clinical practice is yet to be determined.
Genital herpes may be caused by herpes simplex viruses 1 or 2 (HSV-1 or HSV-2) and may be recurrent in up to 20% of those infected. Serological surveys in Australia indicate that about 15% of women attending antenatal clinics and up to 55% of those attending STI clinics have been infected with HSV-2.20 Genital herpes caused by HSV-1 is often transmitted by oral sex, but tends to be milder than that caused by HSV-2 and recurs uncommonly. Some people with evidence of past HSV-2 infection recall no symptoms.20 HSV transmission may uncommonly occur in the absence of genital lesions (risk, about 10% per year). The risk of acquiring genital HSV-2 from a regular partner is greater for women with no previous HSV-1 infection (20% per year) and least for men with previous HSV-1 exposure (5% per year).20
The value of HSV antibody testing in clinical practice is not clearly defined and these tests have, until recently, lacked specificity. The western blot is the recognised gold standard, but is technically demanding, expensive, and not readily available in routine diagnostic laboratories. The sensitivity and specificity of EIA has been improved by use of recombinant viral peptides from HSV-1 and HSV-2, but false positives and false negatives remain a problem with these newer tests, which still do not perform as well as western blots.21 Routine antibody testing is not usually recommended in clinical practice, but when a patient has clinical HSV-2 infection it is useful to know if his or her partner is already infected with HSV-2, as precautions against transmission are then unnecessary.
Aciclovir, valaciclovir and famciclovir are all effective for the treatment of primary and recurrent episodes and for suppression20 (Box 5). The least expensive option is aciclovir (now available in a generic formulation). Primary episodes should be treated regardless of duration of symptoms. On the other hand, symptoms of a recurrence are ameliorated and duration shortened by one to two days if treatment begins within 72 hours of symptom onset. Suppressive therapy prevents at least 75% of attacks and substantially reduces asymptomatic shedding. If recurrent attacks are mild and infrequent then long term suppressive therapy may not be warranted.
The diagnostic approach to syphilis has changed considerably in the past five years. Traditional screening strategies use an initial non-treponemal test (eg, rapid plasma reagin [RPR] or Venereal Disease Research Laboratory [VDRL]), and follow-up positive results with a specific treponemal test (eg, Treponema pallidum particle agglutination or haemagglutination assay, or fluorescent treponemal antibody absorption). However, many laboratories now screen with an automated EIA, which detects treponemal IgG and is therefore equivalent to the specific treponemal tests. EIAs appear to be as sensitive and specific as the traditional tests.22
Unless treatment is instituted in the very early stages of infection, the EIA usually remains positive for life (as does the T. pallidum haemagglutination assay), unlike the non-treponemal tests, which show falling levels (or may become non-reactive) after treatment or spontaneously over time. This means that a small group of people who have been successfully treated for syphilis in the past and have previously had negative RPR results will now test positive on the new screening assays. Conversely, people with untreated syphilis whose RPR has fallen spontaneously to unreactive will now be routinely detected (this group includes up to 25% of patients with neurosyphilis). The cost benefit of the EIA will be less in populations with a high background prevalence of prior syphilis,23 as a significant proportion of people will have a positive EIA result and require further assessment, including an RPR.
In-house PCR tests for syphilis are available in a number of laboratories, but their role in routine diagnosis has not been validated against standard assays. A commercial multiplex PCR test for genital ulcer disease (which tests for T. pallidum, H. ducreyi and HSV) has shown promise in field testing but is yet to be released.24
Penicillin remains the treatment of choice for all stages of syphilis (Box 5). Despite over 50 years of exposure of T. pallidum to this antibiotic, antibiotic resistance does not seem to be a clinical problem.
Patients with penicillin allergy should be desensitised if this can be performed quickly and safely. Ceftriaxone has been used in penicillin-allergic patients, but must be avoided if there is a history of immediate hypersensitivity to penicillin. Although not currently recommended, oral azithromycin has been shown to be effective, and may become more important for syphilis treatment in time.25
Donovanosis is a genito-ulcerative disease caused by a gram-negative organism, Klebsiella granulomatis (formerly Calymmatobacterium granulomatis). It is principally confined to indigenous populations in the tropics,26 and is becoming increasingly rare in Australia since the introduction of azithromycin. The standard diagnostic technique is the identification of Donovan bodies in histological or cytological specimens from lesions. A PCR test has also been developed which is sensitive and specific but is not commercially available.27
Evidence-based recommendations
For treatment of uncomplicated genital chlamydial infection, azithromycin (1 g orally as a single dose) has been shown to be as effective as doxycycline (100 mg orally 12-hourly for 7 days)5 (E2).
Azithromycin (1 g orally once weekly for 4 weeks or 500 mg orally once daily for 7 days) is highly effective treatment for donovanosis and minimises compliance problems28 (E2).
Treatment of asymptomatic trichomoniasis with metronidazole in pregnancy is associated with an increased risk of premature labour. Therefore, preventing trichomoniasis in women of child-bearing age remains a priority (E2).
Minimally invasive testing is acceptable for diagnosis of sexually transmitted infections in women living in remote and rural areas4 (E2).
1: Common presentations of sexually transmitted infections and recommended investigations*
3: Case history — opportunistic screening for sexually transmitted infections
The GP adopted an approach to screening for sexually transmitted infections (STIs) that was appropriate to the age, sexual behaviour and urban residence of the patient. (The approach to symptomatic patients differs and is outlined in Box 1.)
C. trachomatis infection is the most common curable STI affecting young heterosexual people in Australia and has important reproductive sequelae.
Neisseria gonorrhoeae could have been included in the PCR test, but the positive predictive value (proportion of positive tests that truly represent the presence of infection) would have been low because of the low pre-test probability of disease in this young, heterosexual, urban woman.
Similarly, we believe that screening for other STIs need not be routinely offered at the initial consultation with a patient such as this. However, once an STI is diagnosed, HIV testing should be offered. Many clinicians would also test for syphilis and offer hepatitis B vaccination at the first visit.
Pap smears are not recommended for women aged under 18 years unless they have been sexually active for two years or more.
If the GP was unable to contact the girl's sexual contacts, the case should have been referred to the relevant public health authority or sexual health centre.
4: Treatment of sexually transmitted infections (1)*
Chlamydial and other non-gonococcal urethritis and cervicitis
Azithromycin (1 g orally, as a single dose) or doxycycline (100 mg orally, 12-hourly for 7 days).
Ceftriaxone (250 mg intramuscularly) or ciprofloxacin (500 mg orally, as a single dose)
Pelvic inflammatory disease (sexually acquired)
Mild to moderate infection Doxycycline (100 mg orally, 12-hourly for 14 days)
plus either ceftriaxone (250 mg intramuscularly) or ciprofloxacin (500 mg orally, as a single dose).
Severe infection Metronidazole (500 mg intravenously, 12-hourly)
plus doxycycline (100 mg orally, 12-hourly)
plus either cefotaxime (1 g intravenously, 8-hourly) or ceftriaxone (1 g intravenously, daily).
Pelvic inflammatory disease (non-sexually acquired)
Mild to moderate infection Amoxycillin clavulanate (875/125 mg orally, 12-hourly for 7–10 days)
plus doxycycline (100 mg orally, 12-hourly for 7–10 days).
Azithromycin is being investigated as an alternative.
Tinidazole or metronidazole (2 g orally, as a single dose).
Clotrimazole (2% vaginal cream for 3 nights or 500 mg pessary as a single dose).
5: Treatment of sexually transmitted infections (2)*
or imiquimod cream (5%) applied on alternate days for up to 3 months.
or aciclovir (400 mg orally, 3 times daily) for 5 days or until symptoms resolve.
or aciclovir (200 mg orally 8-hourly or 400 mg orally 12-hourly) for up to 6 months.
or benzathine penicillin (1.8 g intramuscularly, as a single dose).
or benzathine penicillin (1.8 g intramuscularly, once-weekly for three doses).
Azithromycin (1 g orally once-weekly for 4 weeks or 500 mg orally once-daily for 7 days).
6: Genital warts
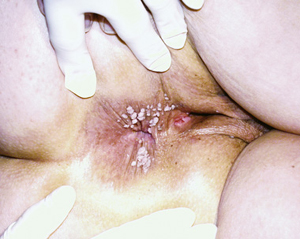 Extensive perianal warts in a 50-year-old woman who had recently begun a new sexual relationship. |
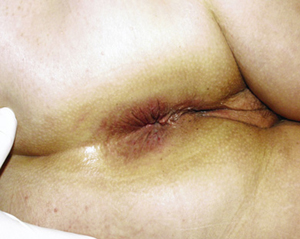 The patient six weeks after beginning a course of topical imiquimod cream (5%), a new immune-response modifier. |
- Francis J Bowden1
- Sepehr N Tabrizi2
- Suzanne M Garland3
- Christopher K Fairley4
- 1 Canberra Sexual Health Centre, Canberra Hospital, Canberra, ACT.
- 2 Microbiology and Infectious Diseases, Royal Women's and Children's Hospital, Melbourne, VIC.
- 3 Melbourne Sexual Health Centre, Melbourne, VIC.
- 1. Communicable Diseases Network Australia — National Notifiable Diseases Surveillance System. Notifications of Chlamydial (NEC) received by State and Territory health authorities in the period of 1991 to 2001 and year-to-date notifications for 2002 by year — month. <http://www.health.gov.au/pubhlth/cdi/nndss/year007.htm> Accessed Apr 2002, no longer available.
- 2. Schacter J. Biology of Chlamydia trachomatis. In: Holmes KK, Sparling PF, Mardh P-A, et al, eds. Sexually transmitted diseases. 3rd ed. New York: McGraw-Hill, 1999..
- 3. Ostergaard L, Andersen B, Moller JK, Olesen F. Home sampling versus conventional swab sampling for screening of Chlamydia trachomatis in women: a cluster-randomized 1-year follow-up study. Clin Infect Dis 2000; 31: 951-957.
- 4. Tabrizi SN, Chen S, Borg AJ, et al. Patient-administered tampon-collected genital cells in the assessment of Chlamydia trachomatis infection using polymerase chain reaction. Sex Transm Dis 1996; 23: 494-497.
- 5. Martin DH, Mroczkowski TF, Dalu ZA, et al. A controlled trial of a single dose of azithromycin for the treatment of chlamydial urethritis and cervicitis. The Azithromycin for Chlamydial Infections Study Group. N Engl J Med 1992; 327: 921-925.
- 6. Stamm W, editor. Chlamydia trachomatis infections of the adult. 3rd ed. Washington: McGraw-Hill, 1999.
- 7. National guideline for the management of non-gonococcal urethritis. Clinical Effectiveness Group (Association of Genitourinary Medicine and the Medical Society for the Study of Venereal Diseases). Sex Transm Infect 1999; 75 Suppl 1: S9-S12.
- 8. Therapeutic Guidelines Ltd. Therapeutic guidelines: antibiotic. Version 11, 2000. Melbourne: Melbourne: Therapeutic Guidelines Ltd, 2000.
- 9. 1998 guidelines for treatment of sexually transmitted diseases. MMWR Morb Mortal Wkly Rep 1998; 47 (No. RR-1): 1-118.
- 10. Kani J, Adler M. Epidemiology of pelvic inflammatory disease. In: Berger GS, Westrom L, editors. Pelvic inflammatory disease. New York: Raven Press, 1992: 7.
- 11. Annual surveillance report: HIV/AIDS, hepatitis C and sexually transmissible infections in Australia. Darlinghurst: National Centre in HIV Epidemiology and Clinical Research, 2000.
- 12. Farrell DJ. Evaluation of AMPLICOR Neisseria gonorrhoeae PCR using cppB nested PCR and 16S rRNA PCR. J Clin Microbiol 1999; 37: 386-390.
- 13. Tapsall J. Annual report of the Australian Gonococcal Surveillance Programme, 1999. Commun Dis Intell 2000; 24: 113-117.
- 14. Bowden FJ, Paterson BA, Mein J, et al. Estimating the prevalence of Trichomonas vaginalis, Chlamydia trachomatis, Neisseria gonorrhoeae, and human papillomavirus infection in indigenous women in northern Australia. Sex Transm Infect 1999; 75: 431-434.
- 15. Patel SR, Wiese W, Patel SC, et al. Systematic review of diagnostic tests for vaginal trichomoniasis. Infect Dis Obstet Gynecol 2000; 8: 248-257.
- 16. Klebanoff MA, Carey JC, Hauth JC, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med 2001; 345: 487-493.
- 17. Koutsky LA, Kiviat NB. Genital human papillomavirus. In: Holmes KK, Sparling PF, Mardh P-A, et al, editors. Sexually transmitted diseases. 3rd ed. New York: McGraw-Hill, 1999: 347-359.
- 18. Koutsky LA. HPV epidemiology — Gollow Lecture. Abstracts of the Australasian Sexual Health Conference – 2001 . . . a sex odyssey; 2-5 May 2001; Sydney, NSW.
- 19. Munoz N. Human papillomavirus and cancer: the epidemiological evidence. J Clin Virol 2000; 19: 1-5.
- 20. Corey L, Wald A. Genital herpes. In: Holmes KK, Sparling PF, Mardh P-A, et al, editors. Sexually transmitted diseases. 3rd ed. New York: McGraw-Hill, 1999: 285-312.
- 21. Ashley RL, Eagleton M, Pfeiffer N. Ability of a rapid serology test to detect seroconversion to herpes simplex virus type 2 glycoprotein G soon after infection. J Clin Microbiol 1999; 37: 1632-1633.
- 22. Egglestone SI, Turner AJ. Serological diagnosis of syphilis. PHLS Syphilis Serology Working Group. Commun Dis Public Health 2000; 3: 158-162.
- 23. Bowden FJ, Bastian I, Johnston F. A community-based approach to the control of sexually transmitted disease in the Northern Territory. Aust N Z J Public Health 1997; 21: 519-523.
- 24. Beyrer C, Jitwatcharanan K, Natpratan C, et al. Molecular methods for the diagnosis of genital ulcer disease in a sexually transmitted disease clinic population in northern Thailand: predominance of herpes simplex virus infection. J Infect Dis 1998; 178: 243-246.
- 25. Gruber F, Kastelan M, Cabrijan L, et al. Treatment of early syphilis with azithromycin. J Chemother 2000; 12: 240-243.
- 26. Richens J. The diagnosis and treatment of donovanosis (granuloma inguinale). Genitourin Med 1991; 67: 441-452.
- 27. Carter J, Bowden FJ, Sriprakash KS, et al. Diagnostic polymerase chain reaction for donovanosis. Clin Infect Dis 1999; 28: 1168-1169.
- 28. Bowden FJ, Mein J, Plunkett C, Bastian I. Pilot study of azithromycin in the treatment of genital donovanosis. Genitourin Med 1996; 72: 17-19.
- 29. Skov SJ, Tait P, Kaldor J, Bowden FJ. A field trial of azithromycin in the treatment of donovanosis. A step towards eradication? Venereology 1997; 11: 11-14.
- 30. Carey JC, Klebanoff MA, Hauth JC, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med 2000; 342: 534-540.





Abstract
Commercially available nucleic acid amplification assays (eg, polymerase or ligase chain reaction) are now the "gold standard" tests for genital chlamydial infection and also have a role in screening for gonococcal infection.
Single-dose oral antibiotics are available for treatment of Chlamydia trachomatis, Neisseria gonorrhoeae and Trichomonas vaginalis infections.
Strains of N. gonorrhoeae in urban Australia are often penicillin resistant, while strains from South East Asia and those in homosexually active men may show high-level resistance to quinolones.
Imiquimod, a novel immune-response modifier, is now available for effective, safe, self-administered treatment of genital warts.
The Pap smear remains the cornerstone of screening for precursor lesions of cervical cancer, but human papillomavirus genotyping may have a role in clinical decision-making for women with equivocal or early precancerous lesions.
Treatment of primary genital herpes changes the clinical course, and long-term suppressive therapy is effective for those with multiple recurrences.