Abstract |
Objective: To ascertain the effectiveness of a home
vaccination service for children behind in their vaccination
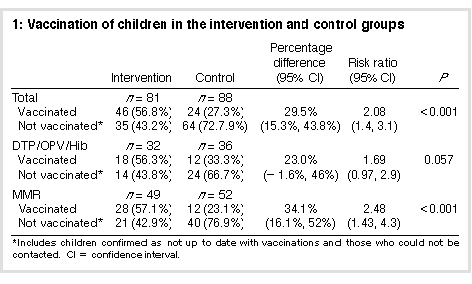
schedule. Design: Randomised controlled trial of nurse-administered vaccination at home. Children were allocated at random to the intervention or the control group before any contact with the parents was made. Setting: 10 council areas in north-west metropolitan Melbourne defined by 56 postcode zones. Six-week intervention period from November 1996. Participants: 405 children -- all those in the study area (n = 2610) 90 days late (age 9 months) for their third diphtheria-tetanus-pertussis/poliomyelitis/Haemophilus influenzae type B (DTP/OPV/Hib) vaccination, or 120 days late (age 16 months) for their measles-mumps-rubella (MMR) vaccination, according to the Australian Childhood Immunisation Register. Main outcome measures: Number of children completing DTP/OPV/Hib or MMR during the intervention period, and number up to date before intervention. Results: Verification of vaccination status with the parents revealed that 123 (60%) of the children in the intervention group and 113 (56%) of those in the control group were up to date with their vaccinations, leaving a study population of 81 (intervention group) and 88 (control group). Vaccination was achieved in 46 (57%) intervention children and 24 (27%) control children (risk ratio [RR], 2.08; 95% CI, 1.4-3.1; P < 0.001). For DTP/OPV/Hib, 18/32 (56%) intervention children and 12/36 (33%) control children were vaccinated (P = 0.06). For MMR, 28/49 (57%) and 12/52 (23%) children were vaccinated, respectively (P < 0.001). Home vaccinations were completed with 26 families (including five siblings). The average cost per child vaccinated as a result of the home program was $92.52. Conclusion: Home vaccination for children behind in their immunisation schedule is an effective, acceptable and relatively cheap method of completing recommended vaccinations. We recommend that a home vaccination program be widely implemented and made available, particularly for disadvantaged families. |
Introduction |
In Australia, incomplete immunisation of children under 2 years of
age remains an important public health problem. Many children never
complete their immunisation schedule or are many months
overdue.1 Uptake rates are
lowest for measles-mumps-rubella vaccination at age 12 months and
the diphtheria-tetanus-pertussis booster at 18 months.1-3 Being late for a primary course is
predictive of being late for or missing subsequent
vaccinations.3,4 Parents' beliefs about the seriousness of the vaccine-preventable illnesses and the safety and efficacy of vaccines are important predictors of vaccination uptake.5 However, there are other barriers to children being vaccinated -- frequent minor childhood illnesses, parental forgetfulness, and advice from health providers to delay vaccinations.6-10 A current strategy to overcome barriers to vaccination is to make vaccination more accessible, but parents are still required to bring their children to be vaccinated. For some families, however, it may be more effective to take the vaccination service to the child. We have explored (i) the effectiveness of offering a home vaccination service to children at greatest risk of not completing their immunisation schedule by age 2 years; and (ii) the usefulness of the Australian Childhood Immunisation Register (ACIR) for identifying these at-risk children. |
Method |
Participants The Australian Childhood Immunisation Register provided identifying information for children living in 10 local council areas in north-west metropolitan Melbourne (defined by 56 postcode zones) who were either born January 1996 and 90 days late for their third diphtheria-tetanus-pertussis/poliomyelitis/Haemophilus influenzae type B vaccination (DTP/OPV/Hib; 1st milestone), or born June 1995 and 120 days late for their measles-mumps-rubella vaccination (MMR; 2nd milestone). The intervention period comprised six weeks from November 1996. Making contact to verify vaccination status before randomisation would have in itself constituted an intervention. Therefore, before any contact was made with the parents, the children were allocated at random (by computer) to the intervention or the control group. Contact We made initial contact with the intervention group by letter, then by telephone one to three weeks later to verify vaccination status, to organise an appointment, and to administer a pre-vaccination health check as recommended by the National Health and Medical Research Council's Australian immunisation handbook.11 This health check was to ensure that the child did not require special medical attention and could be vaccinated at home. If no telephone contact could be made, two follow-up letters were sent. As local councils and maternal and child health nurses provide a substantial number of childhood vaccinations in Victoria and maintain vaccination records, we checked these (possible) providers for vaccination details if parents could not be contacted. Children were confirmed as either overdue for vaccination or up to date with vaccination if parents, the local council or the maternal and child health nurse provided a record of the vaccination. Intervention The study was approved by the Royal Children's Hospital Ethics in Human Research Committee. Parents signed a consent form to participate in the study and a standard State Government vaccination consent form. A nurse administered vaccination in the child's home at a time convenient to the parents. Siblings were also vaccinated if they were due for vaccination. The nurse providing the vaccination had completed a standard Victorian Government Department of Human Services immunisation course. A resuscitation kit (including adrenalin) was taken on each home visit, and the cold chain was maintained by transporting vaccines in a temperature-monitored car refrigerator. Before vaccination, the nurse administered a pre-vaccination health checklist11 to confirm the child's medical history, as obtained during the initial telephone contact, and to assess the child's health on the day of vaccination. Vaccines that were due were verified from the parent-held Child Health Record. The child's temperature was taken if he or she was hot or appeared unwell (a temperature > 38.58c precludes vaccination11). Paracetamol was offered to all children before vaccination. The nurse remained with the family for more than 20 minutes after vaccination. The visits included time for parents to complete questionnaires about immunisation service use, reasons for the delay in vaccination, education level, family size and whether the family had a Health Care Card. (A Health Care Card is a Federal Government card available to low income families, including those receiving government pensions, to obtain concessions for health and medical expenses; ie, it is an indicator of disadvantage or poverty.) Neither written consent nor sociodemographic information was obtained from parents who chose to have their child vaccinated by another provider, or whose child was up to date with immunisation, or who refused to take advantage of the home service. Follow-up of control children Two months after the intervention period, and based on updated information from the Australian Childhood Immunisation Register, we sent letters to parents of control children for whom neither the Register nor local councils had recorded a third DTP/OPV/Hib or an MMR vaccination. We followed the letters with a telephone call to verify vaccination status and to offer, in this case, vaccination at the Royal Children's Hospital. Parents of control children were also informed of local vaccination services offered by the maternal and child health nurse or of the schedules of mobile vaccination vans provided by local councils. No sociodemographic information was collected from the parents of control children. Cost analysis Costs included travel, estimated at $0.50 per kilometre, nursing time at $25 per hour, consumables (excluding vaccines) as charged by the Royal Children's Hospital and clerical work at $17 per hour for 18 days. Statistical analysis Sample size was estimated assuming that 35% of intervention children would accept vaccination and 6% of control children would be immunised. This would require 30 in each group, with a set at 0.05 and statistical power 80%. Statistical associations were assessed with chi-squared tests. Confidence intervals and risk ratios were calculated with the STATA program.12 |
Results |
Subjects There were 2610 children born in June 1995 or January 1996 in the study area and registered with the Australian Childhood Immunisation Register. Of these, 416 children (16%) were identified by the Register as overdue for their third DTP/OPV/Hib or MMR. The Figure shows the number of children on the Register meeting the study criteria, the number excluded and the vaccination status of the children in the intervention and control groups at the time of contact. On verification of vaccination status with parents, 123 (60%) of the intervention children and 113 (56%) of the control children were confirmed as being up to date with their immunisation schedule, and therefore were ineligible for the intervention, leaving 81 children in the intervention group and 88 control children. Those whose status could not be verified were assumed for analysis to be unvaccinated. In total, 2430 (93%) children were up to date with their vaccinations at the beginning of the study period: 1219 (95%; 95% confidence interval [CI], 93.6%-96.0%) 9-month-old children and 1211 (92%; 95% CI; 90.8%- 93.8%) 16-month-old children. Intervention Table 1 shows the number of children vaccinated during the intervention period. To estimate the effect of the intervention on uptake for the full cohort, the cohort was divided into two equal groups (n = 1305 each). The number of children immunised in the group with the intervention children increased from 1220 (93.5%) before intervention to 1266 (97%) after intervention. The group with the control children increased from 1210 (93%) to 1234 (95%). Using similar logic, but dividing for type of vaccine, in the group with intervention children the rate for 1st milestone vaccination increased to 98% and for 2nd milestone to 97%, whereas the rates for the group with control children increased to 96% and 94%, respectively. |
|
| |
|
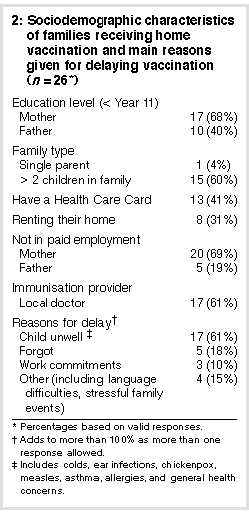
The mean (SD) age for DTP/ OPV/Hib vaccination for intervention and control children was 10 (0.2) months and 11.5 (0.3) months, respectively (which was significantly different; P < 0.001), compared with 7 (1.3) months for children having DTP vaccination before study commencement. The mean (SD) age for MMR vaccination for intervention and control children was 17.2 (0.1) months and 19 (0.3) months (P < 0.001), compared with 14 (1.7) months for those having MMR vaccination before study commencement. In the intervention group 26 children were immunised by the study nurse and 19 by their doctor or local council service. One child who had a severe egg allergy was vaccinated at the Royal Children's Hospital Immunisation Adverse Events Clinic. Ten children due for MMR were also given their 18-month DTP/Hib boosters and five siblings were brought up to date with their vaccination schedule. In all, 82 vaccines were administered to study children and siblings. On the day of vaccination 13 children had colds or were taking antibiotics; none had a fever. All were vaccinated as arranged. Two families refused the service because they were against immunisation and 22 families preferred to use their own doctor. As mentioned, 19 did so within the study period. One mother changed her mind about home vaccination because of concern about her child's egg intolerance. The child was vaccinated a month after the intervention by her doctor. Table 2 summarises the demographic information of those immunised at home and major reasons given by parents for delayed vaccinations.
Costs The mean cost per child vaccinated in the intervention group was $92.52, and the mean cost per visit per vaccine was $52. These costs excluded cost of visits to the general practitioner by those being vaccinated by their own doctor. Fifty-one per cent of the cost was attributable to clerical time needed to verify vaccination status. Travel costs were 12% of total costs and 33% of nurses' costs. | |
Discussion |
We have shown that offering home vaccination is an effective method of
bringing children (and their siblings) up to date with their
immunisation schedule. Importantly, we used information from a
population-based register, and thus provided vaccinations for
children in socially disadvantaged families. Such families have
been identified previously by the Australian Bureau of
Statistics13 and
others14 as being most at
risk of not completing the scheduled childhood immunisations.
A major finding of this study was the unexpectedly high proportion of children already vaccinated at the commencement of the study. This proportion was substantially higher than expected from previous statewide estimates -- 95% v. 84% for 1st milestone vaccination and 92% v. 78% for 2nd milestone vaccination2 -- and exceptionally high given that our study was conducted in a socially disadvantaged area. Data for our study on children's vaccination status were from Australian Childhood Immunisation Register enrolments, which are derived from Medicare data and miss about 2% of children; however, this would have had a minimal impact on these vaccination estimates. It is also unlikely that substantial misclassification of vaccination status occurred. While we did not formally cross-check vaccination dates, when dates were obtained from two sources 85% matched. When dates differed it cannot be assumed that Register dates were correct. In some cases vaccinations reported to the Register by us were incorrectly recorded or missing. Thus, we found the usefulness of the Australian Childhood Immunisation Register as a source of accurate information to be limited. However, our study was undertaken in the first year of the Register's existence and it is expected that accuracy of the Register will improve. Limitations of this study arise from the need to randomise the population sample before verification of immunisation status. This has the potential to introduce bias because of the possibility of differences in response between the intervention and control groups. Another limitation was the number of children in each group with whom no contact was made. However, these limitations are unlikely to have caused substantial bias. In both groups a similar number of control (56%) and intervention children (60%) were excluded because they were up to date with vaccinations, and likewise the proportion of control (15%) and intervention children (14%) who could not be contacted to determine immunisation status was similar. There is also no reason to suppose that any differences in vaccination rates between these two uncontacted groups would be sufficient to bias the estimate of the intervention effect. Assuming that 50% of the children in each group who could not be contacted were vaccinated, the risk ratio for vaccination would be 1.67 (95% CI, 1.3-2.2; P < 0.001). Itinerancy is a risk factor for incomplete and late vaccination,3,15 making it not surprising that a considerable number considered overdue for vaccination could not be contacted. We have shown that those who can be contacted can be vaccinated. A similar program that accesses vaccination information at a local level may be more efficient at targeting families who move frequently. It is obvious that a home service will cost more than mass vaccination programs. The cost per vaccine, taking into account only nurses' time and travel costs, was about $23, which compares favourably with the £8 reported by an outreach program in the United Kingdom.16 The costs of the service would be reduced with improved accuracy of Australian Childhood Immunisation Register information (clerical costs would be reduced by 50%), by offering a local rather than a centralised service (travelling costs would be reduced by 30%), and by incorporating the vaccination service into a broader home visiting program to promote child health and support disadvantaged families in this endeavour. As indicated by many studies,17-22 a barrier to age-appropriate immunisation is often not parental unwillingness to have their child vaccinated, but immunisation providers failing to provide a service. About a third of the parents of children behind in their vaccinations reported having recently consulted a doctor. In almost all these cases the child could have been vaccinated at that time. To prevent diseases such as measles, immunisation rates need to exceed 95%.23 Even the high uptake rates found at the commencement of our study are below this level. Innovative and proactive methods are necessary to attain these high levels and have been found to be effective.15,24 |
Acknowledgements | This study was funded by National Health and Medical Research Council (NHMRC) project grant number HS371. Lyndal Bond was funded by an NHMRC Scholarship. We would like to thank the research nurse (Michelle Wills), the Royal Children's Hospital Immunisation Adverse Events Clinic for providing a service for children in the intervention and control groups, and local council health departments for their cooperation. |
References |
(Received 29 Sep 1997, accepted 6 Apr 1998) |
Authors' details
Clinical Epidemiology and Biostatistics Unit, Department of Paediatrics, University of Melbourne, Royal Children's Hospital, Melbourne, VIC.Lyndal M Bond, MA(ApplPsych), NHMRC Scholar.
Terry M Nolan, PhD, FRACP, FAFPHM, Head, Clinical Epidemiology and Biostatistics Unit.
Department of Human Services, Melbourne, VIC.
Rosemary A Lester, MB BS, MPH, FAFPHM, Head, Infectious
Diseases Unit.
Reprints: Ms Lyndal Bond, Clinical Epidemiology and
Biostatistics Unit, Department of Paediatrics, University of
Melbourne, Royal Children's Hospital, Parkville, VIC 3052.
E-mail: bondATcryptic.rch.unimelb.edu.au
Readers may print a single copy for personal use. No further reproduction or distribution of the articles should proceed without the permission of the publisher. For permission, contact the Australasian Medical Publishing Company
Journalists are welcome to write news stories based on what they read here, but should acknowledge their source as "an article published on the Internet by The Medical Journal of Australia ".
Received 4 March 2026, accepted 4 March 2026
- Lyndal M Bond
- Terry M Nolan
- Rosemary A Lester