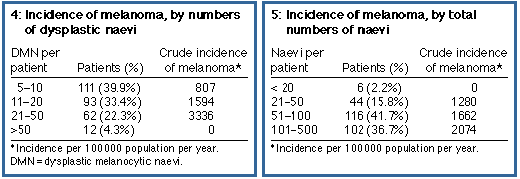A high incidence of melanoma found in patients with multiple dysplastic naevi by photographic surveillance
John W Kelly, Josephine M Yeatman, Cheryl Regalia, Grahame Mason and Amanda P Henham
Readers may print a single copy for personal use. No further reproduction or distribution of the articles should proceed without the permission of the publisher. For permission, contact the Australasian Medical Publishing Company
Journalists are welcome to write news stories based on what they read here, but should acknowledge their source as "an article published on the Internet by The Medical Journal of Australia <http://www.mja.com.au/>".
Abstract - Introduction - Methods - Results - Discussion - References - Authors' details
- - ©MJA1997
Abstract |
Objectives: (1) To assess the incidence of melanoma
in a cohort of patients with dysplastic melanocytic naevi (DMN) and
the relationships between incident melanomas and preexisting naevi
and between melanoma risk and numbers of DMN. (2) To examine the role of
the patient versus the physician in detecting melanoma and the
relative value of surveillance versus prophylactic excision. Design: Prospective cohort study. Patients and setting: Two hundred and seventy-eight adults, each with five or more DMN, were followed up for a mean period of 42 months in a private dermatology practice. DMN were clinically diagnosed. Results: Twenty new melanomas were detected in 16 patients, corresponding to an age-adjusted incidence of 1835/100 000 person-years, 46 times the incidence in the general population. Eleven were detected because of changes evident in comparison with baseline photographs and nine were detected by patients or their partners. Thirteen of the 20 melanomas arose as new lesions and only three from DMN. Melanoma risk rose with increasing numbers of DMN. Conclusions: Increasing numbers of DMN are associated with increasing melanoma risk. Surveillance (baseline photography and follow-up) enabled early diagnosis of melanoma and was very much more cost-effective in preventing life-threatening melanoma than prophylactic excision of DMN. |
Introduction |
Eight case-control studies have indicated that dysplastic
melanocytic naevi (DMN) are a strong and independent risk factor for
the development of melanoma.1-8
Cohort studies show that individuals with DMN develop more
melanomas than the general population.9-14 Only one cohort study has assessed the association of incident melanomas with preexisting naevi and the role of the patient versus the clinician in detection.10 None has examined an Australian cohort or addressed the relationship between DMN numbers and melanoma risk, the cost-effectiveness of surveillance compared with prophylactic excision or the occurrence of non-melanoma skin cancer in subjects with DMN. We followed a cohort of 278 Australian patients with DMN, using baseline skin surface photography to address these issues. |
Methods |
All patients who presented to the private dermatological practice of
one of us (J W K) between March 1985 and November 1992 who required total
body cutaneous examination were assessed for entry to the study. Any
who had five or more clinically determined DMN and who were aged 18
years or more were offered baseline skin surface photography and
follow-up on an annual basis. Over 80% had been referred for
assessment of their atypical naevi or because of other melanoma risk
factors, particularly a personal or family history of melanoma. In
November 1993 all patients who had baseline photographs and had
returned for at least one 12-month follow-up visit were entered in the
study.
The entry criterion of five DMN was based on the results of an earlier study that suggested to us that this number of DMN is associated with sufficient melanoma risk to justify entry to a screening program.2 A systematic set of 14 baseline photographs of the skin surface was used. A clinical photographer (A P H) worked with us in designing the views and did the photography, using anatomical site definitions to define standard views of recognisable body regions rather than using standard magnifications (Box 1). This reduced the number of views needed to cover the skin surface while providing adequate magnification. At entry to the study subjects were advised about their high risk of mela noma; three-monthly self-examination was recommended, the clinical features of early melanoma were discussed and appropriate sun protection measures were described. DMN were diagnosed clinically accor ding to published criteria.15 A naevus was considered clinically dysplastic if it had a macular component and showed at least three of the following five clinical features: ill-defined border, irregularly distributed pigmentation, background erythema, size greater than 5 mm, and irregular border. An exact count was made of the number of DMN and recorded in ranges 1-4, 5-10, 11-20, 21-50 and >50. The total number of MN was estimated and recorded in ranges <20, 21-50, 51-100 and 101-500. Patients were reviewed at 6- to 12- month intervals. Total body cutaneous examination was performed at each visit and the patient's pigmented lesions were compared with baseline photographs. Excisional biopsies were performed for any lesions that were clinically suggestive of melanoma and were not done routinely for histological confirmation of the presence of dysplastic naevi. All histo pathological specimens were examined at a single private pathology laboratory. All melanomas arising during the study were reviewed by one pathologist (G M). Melanomas detected at the initial visit were excluded from the analysis. |
Results |
The study enrolled 278 patients (162 women and 116 men). Mean age was 37
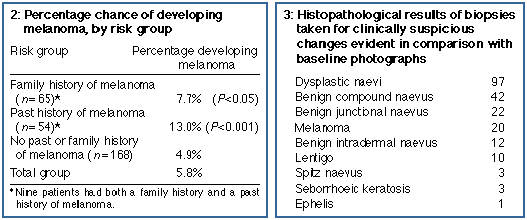
years (range, 18-67 years). Sixty-five patients (23%) had a family
history of melanoma, 54 (19%) had a past history of melanoma, and 9 (3%)
had both. None were from melanoma-prone families (as defined by a
history of two or more affected first degree relatives).
The mean period of follow-up was 42 months (range, 12-99 months). Twenty melanomas were detected in 16 patients over 955 person-years of follow-up. The age-adjusted incidence of melanoma in this group was 1835/100 000 person-years, 46 times the age-adjusted incidence of in-situ and invasive melanoma of 40/100 000 person-years for the population of Victoria in 1990 (G Giles, Anti-Cancer Council of Victoria, 1996, personal communication). The mean time from baseline to diagnosis of melanoma was 36 months (SD, 12 months; range, 11-75 months). Eleven of the 20 melanomas were detected at follow-up visits by comparison with the baseline photographs. These 11 patients were unaware of the changing lesion, and in most of these lesions the change would not have been apparent to the clinician without the use of photo graphs. Seven melanomas were detected by the patient as a change seen on self-examination and two were detected by patients' partners. Four were amelanotic melanomas that did not fulfil the normal diagnostic criteria for melanoma; nonetheless, one of these was detected by the patient. Detecting the 20 melanomas in these 278 patients required 1554 patient consultations (78 for each melanoma). Two hundred and ten biopsies were performed to assess changes observed during follow-up of 104 patients (10 biopsies for each melanoma detected). Not all changes in pigmented lesions led to a biopsy: if melanoma could be confidently ruled out on clinical examination and skin surface microscopy, the lesion was simply rephoto graphed. Histopathological results of these biopsies are shown in Box 3. Changing dysplastic naevi were the predominant source of clinically suspicious change; we do not know how many of these may have progressed to melanoma if they had not been removed. Nineteen non-melanoma skin cancers were detected during follow-up in this group of patients and removed: 16 basal cell carcinomas, one squamous cell carcinoma, one keratoacanthoma, and one Bowen's disease.
Of the 16 patients who developed mela noma, five had a family history and seven had a past history of melanoma. Patients with melanoma were more likely to have had a previous melanoma (P = 0.001) and to have a family history of melanoma (P = 0.05) than those who had not. The percentage of patients in each risk group who developed melanoma is shown in Box 2. Boxes 4 and 5 show the incidence of melanoma in the study cohort in relation to the number of dysplastic naevi and total naevi. For an Australian population, the total numbers of naevi were not remarkable (61% had 100 naevi or less), but there were large numbers of DMN (59% had more than 10). Melanoma incidence correlated more closely with increasing numbers of DMN than with increasing total numbers of naevi. Subjects with 21 to 50 DMN developed melanomas at a rate of 3.3% per year.
The 20 incident melanomas were all superficial spreading in type. Twelve were invasive and eight were in situ. All the invasive lesions were less than 0.6 mm thick and level II,16 except one amelanotic melanoma that showed a desmoplastic component and invaded to 1 mm in thickness and reached level IV.16 According to the criteria of Clark et al.,17 15 were classified as radial growth phase lesions and four as vertical growth phase; one was classified separately as desmoplastic. The melanomas were widely scattered over the skin surface, with six on the upper limbs, four on the upper back, four on the lower limbs, three on the chest, two on the abdomen, and one on the head. Seven melanomas showed histological evidence of an associated benign naevus (intradermal naevoid remnants in four and features of dysplastic naevi in three). Nine melanomas evolved as a change in a preexisting pigmented lesion that had been evident on baseline photos. In two of these nine the preexisting pigmented lesion was likely to have been a de novo melanoma. One of these patients had a stable pigmented lesion on the right side of her chest over six years. It was excised when it began to enlarge and darken and was found to be a level II, 0.6 mm thick melanoma without histological evidence of any associated naevus. A second patient had a tiny, 2 mm diameter lesion on his chest at his first visit. At his initial review visit, six months later, this had enlarged slightly and become more angular in shape. Histologically, this was a level I melanoma with no associated naevus. |
Discussion |
In this study the presence of five or more clinically determined DMN
identified a group of patients with 46 times the general population
incidence of melanoma. None of these patients came from
melanoma-prone families, in whom Clark et al. originally described
dysplastic naevi.18 Five
cohort studies of patients with DMN who are not from melanoma-prone
families have been published and all showed a very high rate of mela
noma.9-13 These findings
confirm those from case-control studies showing that DMN constitute
a strong and independent risk factor for melanoma.1-8 Our age-adjusted incidence of mela noma of 1835/100 000 person-years is higher than that reported in other studies (692-709/100 000 person-years).9,11 This may be explained by the relatively large numbers of DMN in our patients (Box 4) and by the higher background rate of melanoma in Austra lia than in the United States and United Kingdom. We did not examine a cohort of control patients, but Marghoob et al. did.12 They found a 10-year cumulative risk of melanoma of 10.7% among 287 patients with at least one large naevus, one atypical naevus and 100 total naevi. This risk compared with a 0.62% 10-year risk among 831 controls selected from patients requiring annual dermatological follow-up for other reasons. We have intentionally continued to use the word "dysplastic" rather than "atypical". These naevi have been studied because they are believed to be associated with melanoma risk. There are other naevi that are clinically atypical and that are not linked with melanoma risk. Examples are naevus spilus, blue naevus and halo naevus. We prefer the term "dysplastic" because it clearly does not embrace these other atypical naevi. There are several definitions in the literature for syndromes associated with atypical or dysplastic naevi. One includes patients with a single atypical naevus, a large (>8 mm diameter) naevus and >100 total naevi.12 Another depends on distribution of atypical naevi on the anterior scalp, dorsa of the feet, iris or buttocks.19 DMN have been demonstrated to be independently associated with mela noma risk and are not always associated with large numbers of naevi or naevi in certain locations. We prefer not to include DMN in a syndrome definition but rather to consider them as a continuous numerical variable. There is evidence from this study (Box 2) and from two case-control studies of increasing risk with increasing numbers of DMN.2,11 The risk associated with a single DMN is likely to be small and insufficient to justify our follow-up regimen. It is also difficult to accurately diagnose a single naevus as being dysplastic or otherwise on clinical grounds, but much more easy to identify the patient with numerous DMN. It is also difficult to apply definitions of large numbers of naevi in different geographical locations. A recent Australian study reported a mean total count of naevi (>2 mm diameter) that approached 100 in 15-year-old adolescents, which is considered a large number in the United States and United Kingdom.20 The proportion of the Australian adult population with five or more dysplastic naevi has not been defined. One study of 1123 Australian schoolchildren aged 6-15 years showed that 2.7% had three or more dysplastic naevi according to the clinical definition applied here.21 Two-thirds of the incident melanomas in this study were de novo lesions. This finding is similar to those of studies that examine the histological frequency of associated naevi with melanoma, which suggest that 43%-77% of melanomas are new lesions.22-24 The predominance of de novo melanoma in these patients supports management by photographic surveillance rather than by attempts at prophylactic excision. If every one of the 5838 DMN present in our cohort of patients had been excised at the outset, only three of the incident melanomas would have been prevented. Ninety-three DMN were excised in the course of the study because of changes evident in comparison with photographs and because the possibility of melanoma could not be confidently excluded. These changing DMN would seem to be the most likely pigmented lesions to develop into melanoma, and it is possible that some would have progressed to melanoma had they been left in place. When consultations and biopsies are costed at the Australian Government's Medi care Schedule rates for 1997 and $100.00 is allowed per set of photo graphs, the cost of diagnosing each mela noma in this study is $5583. Prophy lactic excision of all dysplastic naevi would have cost $1118038 and would have prevented only three of 20 mela nomas, at a cost of $395038 each. The use of baseline photographs in patients with many floridly atypical pigmented lesions provided knowledge of the stability of many lesions that would otherwise have demanded excisional biopsy, and greatly reduced the number of excisions needed in managing such patients. Patients commonly presented with concern about a change in a particular pigmented lesion. Reference to the photographs usually revealed no change and the lesion remained under observation. Only 10 biopsies were necessary to detect each melanoma and less than one biopsy was necessary per subject in the course of this study. The melanomas incident in this study were detected at an earlier stage than other melanomas that were incident in the State of Victoria in 1990. In-situ melanomas comprised 45% of those seen in the DMN cohort and 33% of those seen in Victoria for 1990 (G Giles, Anti-Cancer Council of Victoria, 1996, personal communication). Median tumour thickness was 0.40 mm in our cohort and 0.77 mm for the State in 1990. Mean tumour thickness for invasive melanoma in the cohort was 0.44 mm compared with 1.40 mm (95% confidence interval, 1.29-1.51) for the State. It is possible that such close surveillance leads to the detection of some melanomas that would otherwise have remained undetected and may have regressed spontaneously or failed to progress and become life threatening. Such "harvesting" of early melanomas may contribute to the very high incidence observed. We conclude that numerous dysplastic naevi identify patients at high risk of melanoma. Baseline photography of the entire skin surface and 6-12-monthly surveillance provides an effective method for early detection of incident melanomas. As most new melanomas were de novo lesions, prophylactic excision of dysplastic naevi would not have provided a satisfactory alternative to follow-up and does not provide sufficient risk reduction to justify the cost and morbidity of the procedure. |
References |
(Received 10 Dec 1996, accepted 10 Jun 1997) |
Authors' details
Victorian Melanoma Service, Alfred Hospital, Melbourne, VIC.John W Kelly, MD BS, FACD, Head; Head of Dermatology Unit, Alfred Hospital; Clinical Associate Professor, Monash University Department of Medicine.
Dermatology Unit, Alfred Hospital, Melbourne, VIC.
Josephine M Yeatman, MB BS, GradDipEpi, Registrar.
Cheryl Regalia, Medical student, University of California at San Diego; now MD.
Dorevitch Laboratories, Melbourne
Grahame Mason, MB BS, FRCPA, Pathologist.
Department of Photography, Northern Territory University, Darwin, NT.
Amanda P Henham, BAppSci(Photog), SRN, Lecturer.
Reprints: Dr J W Kelly, Victorian Melanoma Service, Alfred Hospital, Commercial Road, Prahran, VIC 3181.
©MJA 1997
<URL: http://www.mja.com.au/>
© 1997 Medical Journal of Australia.
Received 28 February 2026, accepted 28 February 2026
- John W Kelly
- Josephine M Yeatman
- Cheryl Regalia
- Grahame Mason
- Amanda P Henham