The known Following the 1991 introduction of the Australian National Cervical Screening Program, the incidence of cervical cancer declined, but trends for histological types in different age groups have not been reported.
The new Squamous cell cancer rates in women aged 25 years or more fell by more than 50%, but have now plateaued among women aged 25–69 years. Screening has had little impact on adenocarcinoma rates in any age group, and there was no decline in cervical cancer rates for 20–24-year-old women.
The implications Our findings support the planned 2017 transition to HPV-based screening starting at age 25, which may also reduce adenocarcinoma incidence.
The National Cervical Screening Program (NCSP) has been very successful in reducing the overall burden of cervical cancer in Australia by facilitating the detection and treatment of pre-cancerous lesions.1 However, in response to new evidence about the optimal age range for screening, new technologies, and the implementation of a successful national human papillomavirus (HPV) vaccination program, a major review of national cervical screening policy (the “renewal”) was recently undertaken.2 Recommended changes to the NCSP include a change from cytology-based screening every 2 years to primary HPV testing every 5 years (including partial HPV genotyping and the referral of HPV 16/18-positive women to colposcopy) and raising the age for starting screening from 18–20 years to 25 years.3,4
Concerns have been expressed about the safety of raising the screening age,5 although the change is consistent with international guidelines6 and with evidence that screening is of limited effectiveness in women under 25 years of age.7 It should also be noted that this change to the starting age is being undertaken in the context of high HPV vaccination coverage in young women in Australia, and of observed reductions in the rates of both high grade cervical abnormalities and of vaccine-included type infections (ie, infections with HPV 6, 11, 16 and 18), including in women who are potentially at higher risk.8-11
Earlier studies have examined how overall rates of cervical cancer have changed in Australia since the introduction of the NCSP,12 but no Australian study has analysed the effect by age group and histological subtype of cancer. Routine screening reports include incidence data classified according to either age or histological type, but not both, and do not include statistical analyses of trends.1 The aim of our study was to examine changes in the incidence of cervical cancer in Australia since the introduction of the current NCSP, taking both age and histological subtype of cervical cancer into account, in order to characterise the impact of the current program before the proposed changes to the NCSP are introduced.
Methods
Data sources
National cervical cancer incidence data for the period 1982–2010 were obtained from the Australian Institute of Health and Welfare. Age-specific rates were calculated, using population estimates from the Australian Bureau of Statistics.13 Three-year average rates were calculated for cervical cancer overall and separately for histological subtypes. The main analyses focused on squamous cell carcinoma (SCC) and adenocarcinoma, but trends for rarer subtypes (adenosquamous and other cancers) were also explored.
Statistical analysis
Standardised rate ratios (SRRs) compared the incidence in each overlapping 3-year period after the introduction of the NCSP with the 3-year average immediately preceding its inception (1988–1990). SRRs and 95% confidence intervals (CIs) were calculated by standard methods14 across all ages, for the target screening group (20–69-year-old women), and for the age groups 25–49, 50–69 and ≥ 70 years. Incidence rate ratios were calculated for the 20–24 years age group (as a single [non-composite] age group it could not be standardised). Joinpoint regression was undertaken to assess whether trends had been consistent over time and to estimate the annual percentage change in incidence. Joinpoint analysis fits the simplest trend model (fewest changes in trends) consistent with the data. To avoid overfitting, we restricted analyses to a maximum of two joinpoints (three trends) across the study period, with the a priori hypothesis that rates declined after the beginning of the NCSP, but allowing for the possibility that this decline had slowed during the second decade of the program, as suggested by visual inspection of the overall rates.
Statistical analysis was performed in SAS 9.3 (SAS Institute) and Joinpoint 4.2.0.2 (Surveillance Research Program, National Cancer Institute [USA]).
Ethics approval
Ethics approval was not required for the study, as only aggregated data were analysed.
Results
During 1982–2010, 26 236 cases of cervical cancer were registered in Australia (SCC, 18 626; adenocarcinoma, 4460; adenosquamous, 1080; other types, 2070). Since 1988–1990, the incidence of cervical cancer has declined, both overall and in all age groups examined, except for women aged 20–24 years. The reductions in incidence between 1988–1990 and 2008–2010 were primarily driven by declines in the rates of SCC (by 50%, 61% and 57% in women aged 25–49, 50–69 and 70 years or more respectively) (Box 1).
Joinpoint analysis indicated that the reduction in the incidence of SCC in women aged 25–49 years occurred mostly during the period 1990–2002 (Box 2, Box 3), without significant change outside this period. For women aged 50–69 years, SCC incidence was dropping prior to the introduction of the NCSP, with a stronger decline between 1994 and 2004, but without change from 2005 (Box 2, Box 3). The change from a decline to a plateau in the incidence of SCC around 2002–2004 was statistically significant for both age groups (P < 0.001). For women aged 70 years or more, the incidence of SCC declined before the introduction of the NCSP, but more rapidly from 1995 (Box 2, Box 3). For women aged 50–69 or 70 years or more, SCC incidence was thus dropping before the NCSP, but the subsequent rates of decrease were greater; these changes in trend were statistically significant (P < 0.001). There were no significant trends in SCC incidence in women aged 20–24 years before the inception of the NCSP, although point estimates suggest an increase until 1986, followed by a decline from 1987 to 1992, then a small, non-significant increase in SCC incidence from 1993. There were similarly no statistically significant trends in the overall incidence of cervical cancer in women aged 20–24 years before or after the start of the NCSP.
The incidence of adenocarcinoma across all ages was 18% lower in 2008–2010 than during 1988–1990. The difference was statistically significant for women aged 25–49 years, but not for other age groups (Box 1). However, there was no consistent downward trend for any age group (Box 4).
Rates of adenosquamous cancer were low, but the relative reduction and timing of the change in its incidence since 1988–1990 were similar to those for SCC in women aged 25–49 and 50–69 years (Appendix, Figures 1 and 2); case numbers among women aged 20–24 years were too small for analysis. The rate of other cervical cancer types appeared to decline across the entire period 1982–2010, without any change that could be related to the beginning of the NCSP, although absolute rates were small and the decline was not significant for women aged 20–24 or 70 years or more (Appendix, Figure 1 and Table 1). Prior to the start of the NCSP, other cervical cancers comprised a larger proportion of cervical cancers in women aged 20–24 years than for other age groups, although their incidence was still very low; they are now extremely rare in this age group (Appendix, Tables 2 and 3).
Discussion
Our analysis confirmed that SCC and overall cervical cancer rates have declined dramatically in women aged 25 years and over since the inception of the NCSP in Australia, but neither has declined in women aged 20–24 years. The overall decline among women aged 70 years or more, who are outside the target age range for screening, was understandably delayed compared with those for women aged 25–49 and 50–69 years, but the overall reduction is now comparable with that in the two younger age groups. While some women continue to be screened after age 69, their number is much smaller than for women in the target age range,1 and screening seems unlikely to explain a reduction in incidence of the magnitude measured. This suggests that the benefits of screening have extended beyond the screening end age in Australia, consistent with case–control data from England and Wales.15
Trends in the incidence of adenocarcinoma since the introduction of organised screening have been less uniform, consistent with findings in other settings of a limited impact of cytology-based cervical screening on adenocarcinoma rates.16,17 This difference has been attributed to the facts that cells from precursor lesions in the endocervical canal are more difficult to sample, and that glandular cells are more difficult to interpret than squamous cells.1
A reduction in the incidence of types of cervical cancer other than SCC was also observed, but appears unconnected with the NCSP, as it occurred throughout the entire study period. It could reflect improvements in clinical follow-up that may have led to improved identification of endometrial cancer that might previously have been misclassified as cervical cancer.
Our findings are timely, given the renewal of the NCSP and the changes in screening policy that will take effect in 2017. After considering the balance of benefits and harms, as recommended by the Australian Screening Framework,18 the Medical Services Advisory Committee (MSAC) recommended that from 2017 women under 25 years of age no longer be screened.3 Our findings support this recommendation: although women aged 20–24 years have been included in the NCSP for more than 20 years, there has been no significant impact on the incidence of either SCC or of cervical cancer overall in this age group. These women also now have a substantially lower risk of cervical cancer because of HPV vaccination. Women under 25 years of age in 2017 will have been offered HPV vaccination at school before they were 15 years old, and the three-dose vaccine uptake rate in these women exceeds 70%.19 The prevalence of vaccine-included HPV types is already very low among women under 25, even among unvaccinated women and woman at potentially higher risk.9-11 High grade cytological abnormality rates have fallen in this age group,8 even though uptake of vaccination in cohorts where this reduction has already been reported was less than 70%, and its efficacy may have been reduced by prior exposure to the virus because these women were vaccinated as young women or older adolescents. Both direct protection and indirect protection for unvaccinated women via herd immunity is likely to be even greater in younger birth cohorts:9 direct protection because females vaccinated as younger adolescents have higher vaccination coverage and lower rates of HPV exposure prior to vaccination; indirect protection because more of the population has since been vaccinated, and because boys are now offered vaccination.20
While screening women under 25 years of age does not appear to substantially affect the incidence of cervical cancer among 20–24-year-old women, it is possible that it might reduce cancer rates among women aged 25–29 years by detecting and treating pre-cancerous lesions before the age of 25. This possibility could not be directly assessed in our study, but data from a UK case–control study suggest that being screened between the ages of 22 and 24 years does not reduce the risk of cancer for women aged 25–29 years.7 An important change to the NCSP from 2017 is that women will receive explicit invitations to attend screening, receiving the first close to their 25th birthday. A switch from a reminder-based to an invitation-based program was a key recommendation of the MSAC,3 and modelling indicates this change will have an important impact on the effectiveness of the program in young women, and on the program overall.21 A wide range of program designs were modelled, and it was estimated that inviting women at age 25 would reduce cervical cancer incidence across all ages by about 2% compared with an otherwise identical program without invitations (in which case screening would probably commence more gradually between the ages of 25 and 29 years).21
An alternative explanation for our finding that the incidence of SCC and of cervical cancer overall in women aged 20–24 years did not decrease after the start of the NCSP is that the impact of the program has been limited by falling screening participation in this age group. However, while their 2-year participation rates have fallen since reporting began (1996–1997), there have been similar falls in participation rates for women aged 25–29 and 30–34 years, among whom cancer rates have declined.22 Additionally, participation by women aged 20–24 years has mainly fallen since 2006–2007, and this would be unlikely to have affected our findings, because the reductions in cancer incidence in other age groups predominantly occurred within the first 10–15 years of the organised screening program, with little change in rates in recent years.
Another possibility is that screening women aged 20–24 years has suppressed a rise in cervical cancer that would otherwise have followed a hypothetical increase in risk behaviour among young women (eg, first intercourse or more sexual partners at a younger age). The results of sexual behaviour surveys are inconclusive as to whether such an increase has occurred, and therefore about whether it would affect our findings. Data from a national population-based survey of sexual behaviour indicate that the median age at first vaginal intercourse was the same for women aged 20–24 years before and immediately after the start of the NCSP (ie, women born 1965–1974) as it was for women who were 20–24 years old during the remainder of period covered by our analysis (ie, women born 1975–1990).23 However, this study also reported a difference between these cohorts in the proportion of women who reported first intercourse before the age of 16 years (a rise from 12.7% to 18.2%).23 There are no data on the number of sexual partners before the age of 20 years that would allow a comparison of these cohorts. Although we cannot exclude an increase in risk behaviour, it seems unlikely that it would fully explain the observed stable incidence of cervical cancer among 20–24-year-old women because, given the magnitude of the reductions in rates in other age groups, it presupposes that a major increase in risk behaviour coincided with the period of the NCSP.
A limitation of our analysis is that cervical cancer incidence rates reported here were not adjusted for hysterectomy rates, as data for this factor were not available for the entire study period. This limitation is common to all routine reports on cervical cancer incidence in Australia,8 but it means that the denominator does not perfectly reflect the true population at risk of cervical cancer. We may therefore have underestimated the incidence of cervical cancer in older women. However, this limitation would not affect our findings for women aged 20–24 years, and would probably have only a small impact on our findings for those aged 25–49 years. Reductions in rates for older women may have been overestimated if part of the drop was attributable to rates of hysterectomy increasing since the mid-1990s, but survey data suggest that they did not.24,25 Further, as the overall reductions in cancer incidence were substantial, they are unlikely to be fully explained by changes in hysterectomy rates.
The current NCSP has been highly successful in reducing the incidence of squamous cervical cancer in Australia, by at least 50% in women aged 25 years or more. However, its effectiveness has been limited among women under 25, and in reducing adenocarcinoma rates. Further, as participation in screening has plateaued (and is falling in some age groups), cervical cancer incidence also appears to have plateaued, if at a lower level than before the program. The National HPV Vaccination Program and the renewed NCSP have the potential to mitigate these limitations. HPV vaccination will provide protection for younger women, while both HPV vaccination and HPV-based screening are expected to reduce adenocarcinoma rates.26 It is estimated that the renewed NCSP will reduce cervical cancer incidence and mortality by at least a further 20%,3,4,21 assuming that active invitations and recalls are effective in achieving high participation rates. In the longer term, the combination of HPV vaccination and the renewed NCSP is predicted to reduce cervical cancer incidence by about 70% below what would have been expected without program change and vaccination,4 and will therefore further reduce the burden of cervical cancer among women in Australia.
Box 1 – Cervical cancer incidence (per 100 000 women) and standardised rate ratios (SRRs) comparing the 3-year average incidence of cervical cancer during 2008–2010 with the 3-year average incidence immediately prior to inception of the National Cervical Screening Program (1988–1990), by histological cancer type and age group
|
Squamous cell carcinoma |
Adenocarcinoma |
All cervical cancer |
|||||||||||||
1988–1990 |
2008–2010 |
SRR (95% CI) |
1988–1990 |
2008–2010 |
SRR (95% CI) |
1988–1990 |
2008–2010 |
SRR (95% CI) |
|||||||
All ages |
9.9 |
4.5 |
0.46 (0.43–0.49) |
2.0 |
1.6 |
0.82 (0.72–0.93) |
13.5 |
7.0 |
0.51 (0.49–0.54) |
||||||
20–69 years |
13.2 |
6.1 |
0.46 (0.43–0.50) |
2.7 |
2.3 |
0.84 (0.73–0.96) |
18.0 |
9.3 |
0.52 (0.49–0.55) |
||||||
20–24 years* |
1.5 |
1.3 |
0.91 (0.55–1.51) |
0.4 |
0.4 |
0.91 (0.35–2.40) |
2.6 |
1.8 |
0.70 (0.46–1.05) |
||||||
25–49 years† |
13.4 |
6.7 |
0.50 (0.46–0.55) |
3.2 |
2.7 |
0.83 (0.70–0.97) |
18.9 |
10.3 |
0.55 (0.51–0.59) |
||||||
50–69 years† |
16.7 |
6.6 |
0.39 (0.35–0.45) |
2.6 |
2.2 |
0.86 (0.67–1.10) |
21.7 |
10.0 |
0.46 (0.42–0.51) |
||||||
≥ 70 years† |
16.8 |
7.2 |
0.43 (0.36–0.51) |
2.8 |
2.0 |
0.71 (0.50–1.02) |
22.7 |
11.4 |
0.50 (0.43–0.58) |
||||||
* Results presented as age-specific incidence rates and age-specific incidence rate ratios. † Results presented as age-standardised incidence rates and standardised rate ratios, using the Australian 2001 Standard Population.13 | |||||||||||||||
Box 2 – Three-year average cervical cancer incidence (with 95% CIs), by age and histological type, 1982–2010
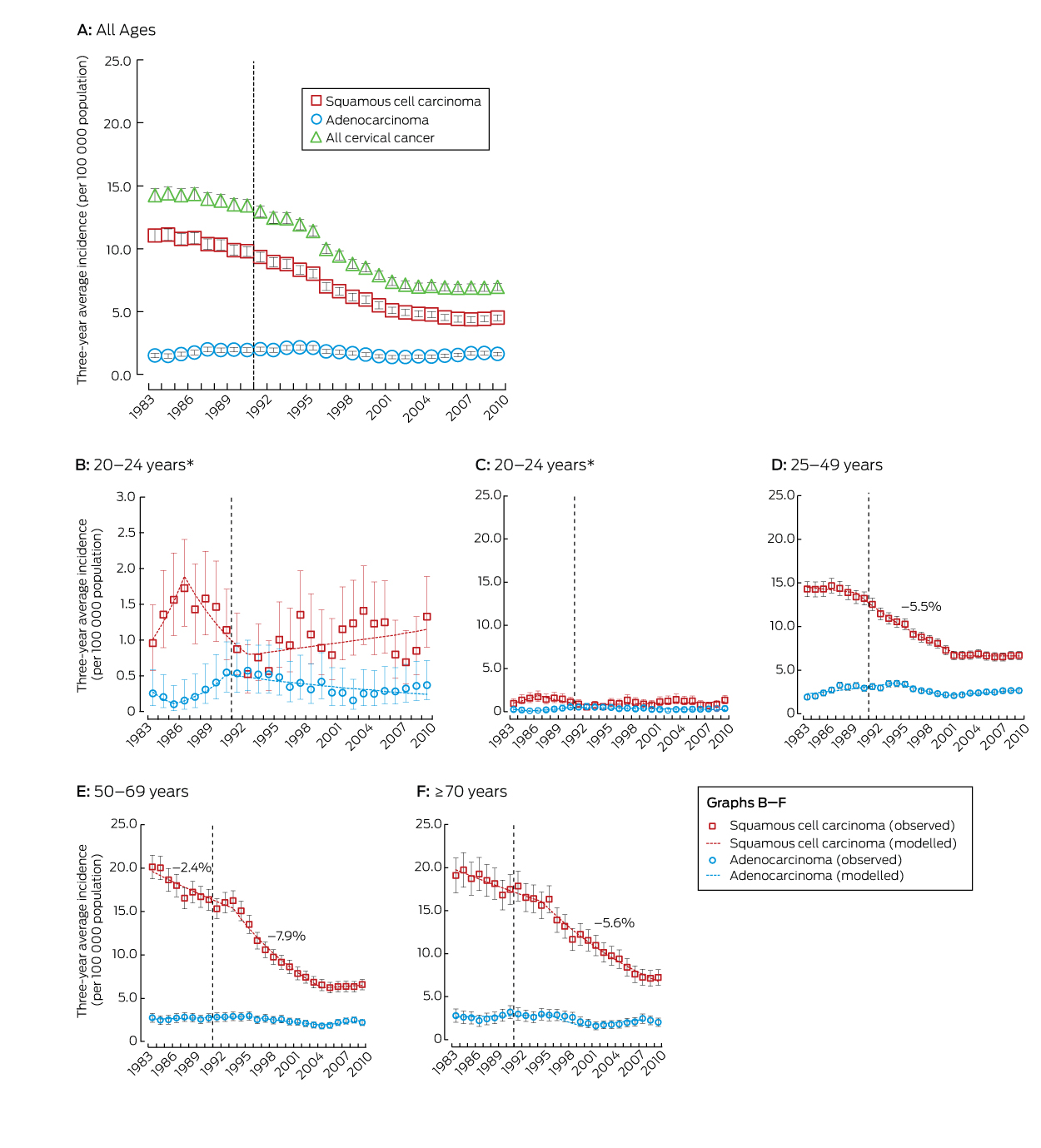
The dashed line represents the start of the National Cervical Screening Program in Australia. Rates for all ages and for the age groups 25–49, 50–69 and ≥ 70 years were standardised, using the Australian 2001 Standard Population.13 The annual percentage changes in incidence rates are included for periods when the change was significant at P < 0.05. * Results for the age group 20–24 years are depicted twice; the vertical axis scale in panel C is the same as for the other age groups, to assist comparison, while panel B uses a compressed vertical axis for clearer display.
Box 3 – Annual percentage change in the 3-year average incidence of squamous cell carcinoma, by age
Age group/period |
Annual change in incidence (95% CI) |
P |
|||||||||||||
20–24 years |
|||||||||||||||
1983–1986 |
23.1% (–14.2% to 76.6%) |
0.24 |
|||||||||||||
1987–1992 |
–13.4% (–26.3% to 1.7%) |
0.08 |
|||||||||||||
1993–2009 |
2.2% (–0.4% to 4.8%) |
0.09 |
|||||||||||||
25–49 years |
|||||||||||||||
1983–1989 |
–0.8% (–1.7% to 0.2%) |
0.12 |
|||||||||||||
1990–2002 |
–5.5% (–5.9% to –5.2%) |
< 0.001 |
|||||||||||||
2003–2009 |
–0.01% (–1.0% to 0.9%) |
0.99 |
|||||||||||||
50–69 years |
|||||||||||||||
1983–1993 |
–2.4% (–3.1% to –1.7%) |
< 0.001 |
|||||||||||||
1994–2004 |
–7.9% (–8.7% to –7.1%) |
< 0.001 |
|||||||||||||
2005–2009 |
0.9% (–1.8% to 3.6%) |
0.5 |
|||||||||||||
≥ 70 years |
|||||||||||||||
1983–1994 |
–1.9% (–2.6% to –1.1%) |
< 0.001 |
|||||||||||||
1995–2009 |
–5.6% (–6.1% to –5.1%) |
< 0.001 |
|||||||||||||
Box 4 – Incidence rate ratios (with 95% CIs) comparing the 3-year average cervical incidence with the 3-year average immediately before the start of the National Cervical Screening Program (1988–1990), by histological type and age
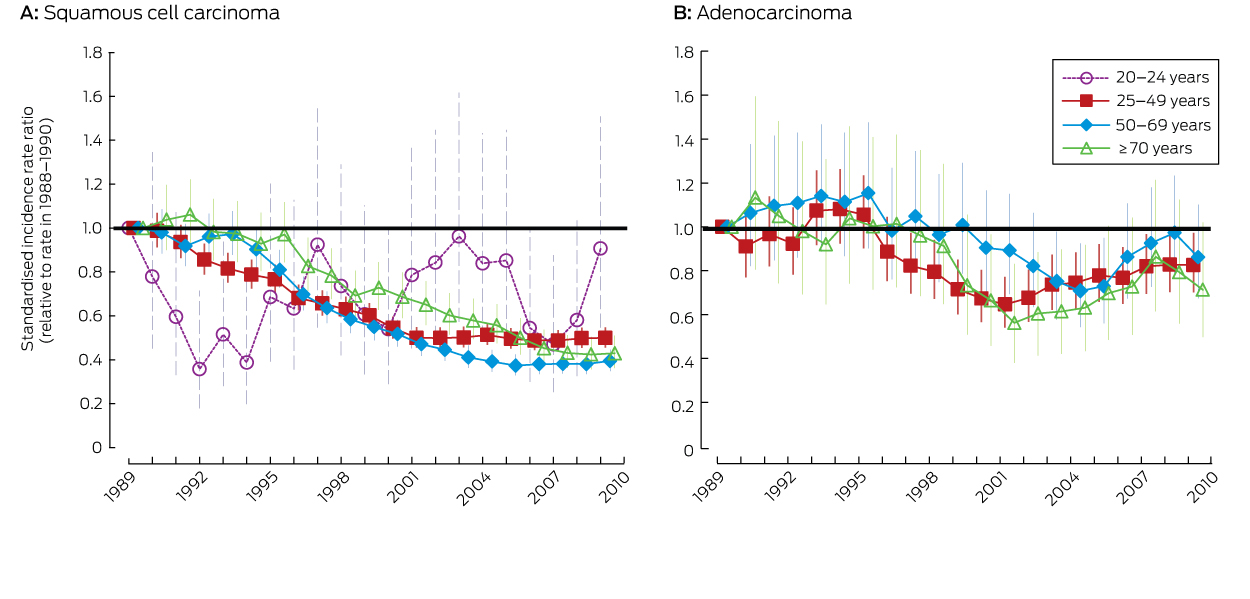
Data for the age groups 25–49, 50–69 and ≥ 70 years are presented as standardised rate ratios, using the Australian 2001 Standard Population.13 Data for the 20–24 years age group are presented as age-specific incidence rate ratios.
Received 10 March 2016, accepted 24 May 2016
- Megan Smith1,2
- Karen Canfell1,2
- 1 Cancer Council NSW, Sydney, NSW
- 2 University of Sydney, Sydney, NSW
We thank the Australian Institute of Health and Welfare Cancer and Screening Unit for providing data from the Australian Cancer Database for this study. We thank Yoon Jung Kang for repeating and checking our statistical calculations. Karen Canfell receives salary support from the National Health and Medical Research Council (Career Development Fellowship APP1082989). Megan Smith was awarded funding by the University of Sydney Postgraduate Research Support Scheme to partially reimburse travel expenses incurred to present these (and other) research findings at HPV2015 (Lisbon, 2015).
Karen Canfell is co-principal investigator of an investigator-initiated trial of cytology and primary HPV screening in Australia (“Compass”; ), which is conducted and funded by VCS, a government-funded health promotion charity. VCS has received equipment and a funding contribution for the Compass trial from Roche Molecular Systems and Ventana USA. Karen Canfell is also a Principal Investigator on “Compass NZ” (ACTRN12614000714684), which is conducted and funded by Diagnostic Medlab (DML; nowAuckland District Health Board). DML received equipment and funding contributions for the Compass trial from Roche Molecular Systems. Neither Karen Canfell nor her institution (Cancer Council NSW) on her behalf received direct funding from industry for this trial or any other project.
- 1. Australian Institute of Health and Welfare. Cervical screening in Australia 2011–2012 (AIHW Cat. No. CAN 79; Cancer Series No. 82). Canberra: AIHW, 2014. http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=60129547030 (accessed July 2016).
- 2. Australian Government Department of Health. National Cervical Screening Program: overview of the renewal. 2014. http://www.cancerscreening.gov.au/internet/screening/publishing.nsf/Content/overview-of-the-renewal (accessed Jan 2015).
- 3. Australian Government, Medical Services Advisory Committee. MSAC outcomes. Application No. 1276 – Renewal of the National Cervical Screening Program. Apr 2014. http://www.msac.gov.au/internet/msac/publishing.nsf/Content/D924E2F768B13C4BCA25801000123B9E/$File/1276%20-%20Final%20MSAC%20PSD%20-%20NCSP%20Renewal.pdf (accessed Aug 2016).
- 4. Cancer Council Australia, Cervical Cancer Prevention Guidelines Working Party. Draft clinical management guidelines for the prevention of cervical cancer. Sydney: Cancer Council Australia, 2016. http://wiki.cancer.org.au/australia/Guidelines:Cervical_cancer/Prevention (accessed Feb 2016).
- 5. Farnsworth A. Changes to cervical screening in Australia: applying lessons learnt. Med J Aust 2014; 201: 245-246. <MJA full text>
- 6. International Agency for Research on Cancer. Cervix cancer screening (IARC Handbooks of Cancer Prevention, volume 10). Lyon: IARC Press, 2005. http://screening.iarc.fr/doc/HANDBOOK10.pdf (accessed July 2016).
- 7. Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ 2009; 339: b2968.
- 8. Australian Institute of Health and Welfare. Cervical screening in Australia 2012–2013 (AIHW Cat. No. CAN 91; Cancer Series No. 93). Canberra: AIHW, 2015. http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=60129550872 (accessed July 2016).
- 9. Tabrizi SN, Brotherton JM, Kaldor JM, et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis 2014; 14: 958-966.
- 10. Osborne SL, Tabrizi SN, Brotherton JM, et al. Assessing genital human papillomavirus genoprevalence in young Australian women following the introduction of a national vaccination program. Vaccine 2015; 33: 201-208.
- 11. Chow EP, Danielewski JA, Fehler G, et al. Human papillomavirus in young women with Chlamydia trachomatis infection 7 years after the Australian human papillomavirus vaccination programme: a cross-sectional study. Lancet Infect Dis 2015; 15: 1314-1323.
- 12. Simonella L, Canfell K. The impact of a two- versus three-yearly cervical screening interval recommendation on cervical cancer incidence and mortality: an analysis of trends in Australia, New Zealand, and England. Cancer Causes Control 2013; 24: 1727-1736.
- 13. Australian Bureau of Statistics. 3101.0 Australian demographic statistics, Mar 2013 [website]. Canberra: ABS, 2013. http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3101.0Mar%202013?OpenDocument (accessed Apr 2014).
- 14. Boyle P, Parkin DM. Statistical methods for registries. In: Jensen OM, Parkin DM, MacLennan R, et al, editors. Cancer registration: principles and methods. Lyon: International Agency for Research on Cancer, 1991; pp 126-158. https://www.iarc.fr/en/publications/pdfs-online/epi/sp95/SP95.pdf (accessed July 2016).
- 15. Castañón A, Landy R, Cuzick J, Sasieni P. Cervical screening at age 50–64 years and the risk of cervical cancer at age 65 years and older: population-based case control study. PLoS Med 2014; 11: e1001585.
- 16. Bergström R, Sparén P, Adami HO. Trends in cancer of the cervix uteri in Sweden following cytological screening. Br J Cancer 1999; 81: 159-166.
- 17. Oh CM, Jung KW, Won YJ, et al. Trends in the incidence of in situ and invasive cervical cancer by age group and histological type in Korea from 1993 to 2009. PLoS One 2013; 8: e72012.
- 18. Australian Population Health Development Principal Committee, Screening Subcommittee. Population based screening framework. Canberra: Australian Health Ministers’ Advisory Council, 2008. http://www.cancerscreening.gov.au/internet/screening/publishing.nsf/Content/16AE0B0524753EE9CA257CEE0000B5D7/$File/Population-based-screening-framework.PDF (accessed July 2016).
- 19. National HPV Vaccination Program Register. Coverage data [website]. 2015. http://www.hpvregister.org.au/research/coverage-data (accessed Aug 2015).
- 20. Smith MA, Lew JB, Walker RJ, et al. The predicted impact of HPV vaccination on male infections and male HPV-related cancers in Australia. Vaccine 2011; 29: 9112-9122.
- 21. Lew JB, Simms K, Smith MA, et al. National Cervical Screening Program Renewal: effectiveness modelling and economic evaluation in the Australian setting. MSAC application number 1276. Assessment report. Canberra: Australian Government Department of Health, 2014. http://www.cancerscreening.gov.au/internet/screening/publishing.nsf/Content/E6A211A6FFC29E2CCA257CED007FB678/$File/Renewal%20Economic%20Evaluation.pdf (accessed July 2016).
- 22. Australian Institute of Health and Welfare. Cervical screening in Australia 2012–2013 (supplementary tables). May 2015. http://www.aihw.gov.au/publication-detail/?id=60129550871&tab=3 (accessed Dec 2015).
- 23. Rissel C, Heywood W, de Visser RO, et al. First vaginal intercourse and oral sex among a representative sample of Australian adults: the Second Australian Study of Health and Relationships. Sex Health 2014; 11: 406-415.
- 24. Australian Institute of Health and Welfare. Cervical screening in Australia 1997–1998. Canberra: AIHW, 2000. http://www.aihw.gov.au/publication-detail/?id=6442467177 (accessed July 2016).
- 25. Australian Institute of Health and Welfare. Cervical screening in Australia 2001–2002. Canberra: AIHW, 2004. http://www.aihw.gov.au/publication-detail/?id=6442467658 (accessed July 2016).
- 26. Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet 2014; 383: 524-532.





Abstract
Objectives: The Australian National Cervical Screening Program (NCSP) will transition in 2017 from cytology-based screening every two years, starting from age 18–20 years, to HPV-based screening every 5 years, starting from age 25. To examine the impact of the program before this transition we analysed trends in the incidence of cervical cancer, by age and histological subtype.
Design, setting and participants: National cervical cancer incidence data, 1982–2010.
Main outcome measures: Standardised rate ratios (SRR) for 3-yearly average cervical cancer incidence, relative to the rate during 1988–1990, by age group and histological type.
Results: Between 1988–1990 and 2008–2010, cervical cancer incidence fell substantially in women aged 25–49 (SRR, 0.55; 95% CI, 0.51–0.59), 50–69 (SRR, 0.46; 95% CI, 0.42–0.51) and 70 years or more (SRR, 0.50; 95% CI, 0.43–0.58), but not in women aged 20–24 years (SRR, 0.70; 95% CI, 0.46–1.05). These declines were primarily driven by drops in squamous cell carcinoma (SCC) in women aged 25–49 (SRR, 0.50; 95% CI, 0.46–0.55), 50–69 (SRR, 0.39; 95% CI, 0.35–0.45) and more than 70 years (SRR, 0.43; 95% CI, 0.36–0.51). However, rates have now plateaued in women aged 25–69 years. The incidence of adenocarcinoma did not consistently decline across the program period in any age group. The incidence of neither SCC (SRR, 0.91; 95% CI, 0.55–1.51) nor adenocarcinoma (SRR, 0.91; 95% CI, 0.35–2.40) declined in women aged 20–24 years.
Conclusion: Although women aged 20–24 years have been included in the NCSP since its inception, no significant impact on cervical cancer incidence was observed in this age group. The NCSP has had a substantial impact on SCC and overall cervical cancer incidence in women aged 25 years and over. Its impact on the incidence of adenocarcinoma, in contrast, has been limited.