Iodine is an essential micronutrient that plays a crucial role in ensuring the normal development of most organs, especially the brain.1-3 The World Health Organization reports that even moderate iodine deficiency can cause a loss of 10–15 points in intelligence quotient, and iodine deficiency is the world’s greatest single cause of preventable brain damage and developmental delay.4 Lack of iodine in the diet, or iodine deficiency disorder, is a major individual and public health problem in many regions of the world. Pregnant women and their babies are particularly vulnerable to iodine deficiency. The WHO global database on iodine deficiency lists Australia as one of the world’s 54 iodine-deficient nations.5
To address Australia’s iodine deficiency, Food Standards Australia New Zealand (FSANZ) has recently introduced a mandatory, Australia-wide, iodine supplementation program through bread products. Although this supplementation program is based on strong evidence of iodine deficiency from a small number of regional urinary iodine excretion studies, there has been no Australia-wide presupplementation baseline data study (the only national iodine nutrition study was conducted in 2003–2004).6 In addition, although the Australian Institute of Health and Welfare has recommended postsupplementation monitoring with the urinary iodine excretion method, no Australia-wide monitoring program has yet been announced.7
Routine neonatal blood screening for metabolic and genetic disorders in Australia and many other countries includes the determination of neonatal TSH concentrations. In five separate large studies in Switzerland, Poland, Bulgaria, Belgium and Thailand, it was found that TSH monitoring could offer an effective method for monitoring population iodine status.8-12 The author of a recent review urged that a confirmation study of the sensitivity of this method of monitoring iodine fortification be carried out in an iodine-sufficient country with a newborn screening program.13 Our study provides baseline information on the iodine status of the neonatal population of Victoria that will allow comparison with the period after iodine fortification in Victoria. Victorian neonatal TSH concentrations are consistent with previous studies indicating population iodine deficiency.6,14
In Australia, heel-prick blood samples are collected routinely from newborns at 2–4 days of age, and the TSH concentration is determined by enzyme-linked immunosorbent assay (ELISA).15 Since 1977, blood TSH concentrations of Victorian newborns have been determined at the laboratory of the Victorian Clinical Genetics Services (VCGS) and recorded on a database arranged according to the postcode of the birth hospital. The latest audit of compliance with the heel-prick test was conducted in 2002, and showed that more than 99.4% of Victorian neonates were screened for TSH concentration.16 The TSH test kit used was the Elegance neonatal TSH ELISA kit (Bioclone Australia, Sydney, NSW) and the samples were analysed using a Labsystems Multiskan RC plate reader (Labsystems, Helsinki, Finland).
Victorian neonatal TSH values, birth hospital postcode, sex, date of birth, time of taking the blood sample and twin information from the past 30 years was extracted from the Victorian neonatal database. Complete full-year neonatal TSH data analysis was performed for the 6 years 2001–2006. The total number of individual neonatal blood TSH samples analysed during the period was 379 735. We excluded 11 183 individual neonatal TSH values because they were repeat sample tests or from blood samples that had not been taken within 96 hours of birth. This left data for 368 552 neonates, representing about 61 425 tests per year over the period. According to the 2007 annual report of the Consultative Council on Obstetric and Paediatric Mortality and Morbidity, the total number of births in Victoria in 2001–2006 was 385 773.17 Thus, our sample of 368 552 individual neonatal TSH values represents 95.5% of the total neonatal population.
De-identified neonatal TSH values were analysed for iodine deficiency on the basis of WHO/United Nations Children’s Fund (UNICEF)/International Council for the Control of Iodine Deficiency Disorder (ICCIDD) criteria, whereby more than 3% of neonatal blood samples taken 3–4 days after birth having a TSH concentration over 5 mIU/L is indicative of an iodine-deficient population.4 The percentage of neonatal TSH concentrations greater than 5 mIU/L for each year was calculated. The data were arranged by birth-hospital postcode and then regrouped into the nine geographical regions designated by the Victorian Department of Human Services (separating northern and western metropolitan regions) using SAS Enterprise Guide software, version 4.1 (SAS Institute Inc, Cary, NC, USA), and ArcMap, version 9.3 (Esri Inc, Redlands, CA, USA) was used as mapping software. The χ2 test was used to determine the statistical difference in the frequency distribution of TSH concentrations greater than 5 mIU/L, and a P value of < 0.05 was considered statistically significant.
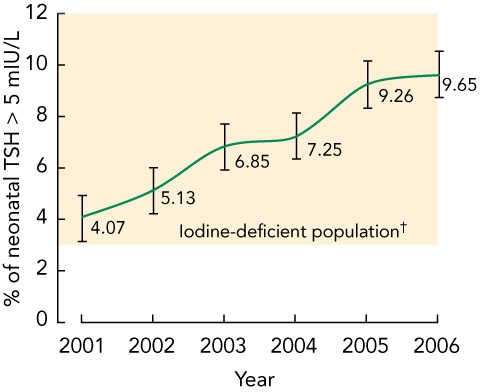
The mean percentage of TSH concentrations above 5 mIU/L in Victorian neonates ranged from 4.07% in 2001 to 9.65% in 2006 (Box 1), indicating an iodine-deficient status according to the WHO, UNICEF and ICCIDD criteria, for that period. This more than twofold increase over 5 years was statistically significant for 2001–2006, and for each yearly increase in that period (P < 0.001).
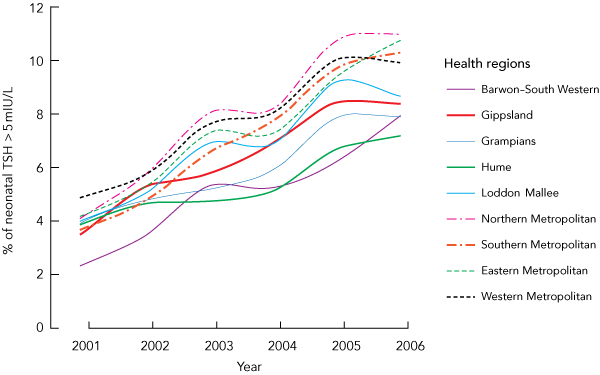
Neonatal TSH values were grouped into the nine Department of Human Services health regions in Victoria and the percentage of TSH concentrations above 5 mIU/L were replotted (Box 2). The percentage of neonatal TSH concentrations above 5 mIU/L in Gippsland increased from 3.54% in 2001 to 8.37% in 2006, and this more than twofold increase was statistically significant (P < 0.001). However, our analysis indicated that the iodine status of the population in all nine Department of Human Services health regions in Victoria had worsened over the period 2001–2006 (Box 1 and Box 2). Among the regions, the metropolitan areas all had a higher percentage of newborns with blood TSH concentrations above 5 mIU/L than the non-metropolitan areas, and this was statistically significant (P < 0.05). The Northern Metropolitan region had the highest frequency of neonatal TSH values above 5 mIU/L, at 11.01% in 2006. Within each region, the differences between the years 2001 and 2006 were statistically significant (P < 0.001). Additionally, each yearly increase in that period for each region was statistically significant (P < 0.05), and the difference between regions was statistically significant (P < 0.05).
Our analysis of neonatal TSH concentrations, representing 95.5% of Victorian children born during 2001–2006, indicates that the Victorian population is iodine deficient according to WHO, UNICEF and ICCIDD criteria. This result is consistent with three smaller Australian studies in Sydney in New South Wales that used newborn TSH data.18-20 One Sydney study found that 8.1% of newborns born between August 1998 and April 1999 (1316) and 5.5% of newborns born between 1 March and 31 December 2000 (1457) had TSH values above 5 mIU/L.18 The second Sydney study, carried out in 2002 and 2003 found that 7.1% of newborns born by caesarian section (651) and 4.3% of newborns born by vaginal delivery (1380) had TSH levels above 5 mIU/L.19 The third Sydney study, carried out in 2005 and involving 815 pregnant women and 824 newborns, found only 2.2% of newborns (18) with TSH values above 5 mIU/L; however, the results of the urinary iodine excretion study of the 815 pregnant women indicated iodine deficiency.20 Unlike our study, these studies were based on small sample numbers collected over a short period (less than a year).
Our findings are also consistent with those of two further urinary iodine excretion studies — the 2003–2004 National Iodine Nutrition Study (NINS), which included 348 Victorian schoolchildren,6 and a 2001 study of 577 schoolchildren in Melbourne, Victoria.14 Both studies found that Victorian schoolchildren had urinary iodine excretion concentrations indicative of mild iodine deficiency according to WHO, UNICEF and ICCIDD criteria.
Some factors that might affect newborn TSH concentrations include age at the time of collection of blood samples, thyroid hormone supplementation during pregnancy and exposure to iodine-containing disinfectants during delivery. Effects from iodine-based disinfectants during delivery or thyroid hormone therapy during pregnancy could not be ruled out, as these data were not recorded in the neonatal database. However, the data analysis followed the standard guidelines for age at collection of blood samples and only included blood samples that were taken at between 48 and 96 hours from birth.21-24 WHO, UNICEF, ICCIDD and national food standards authorities, such as FSANZ, have emphasised the need for regular monitoring of population iodine status. The urinary iodine excretion assay, although recognised as the gold standard test, is expensive in terms of time, cost, personnel and effort. We suggest that the regular analysis of routine neonatal screening TSH test results is an easier and less expensive method of monitoring iodine deficiency in the most vulnerable population group (pregnant women). Neonatal TSH data are collected as part of an ongoing national population screening program for congenital diseases, and so provide an opportunity for regular and continual monitoring of this population’s iodine status. This approach has been used very effectively to monitor the beneficial effects of the Swiss iodised salt program.9 An additional advantage with this method is its flexibility as the monitoring may be carried out on an ongoing basis as part of a national, state-by-state or regional monitoring program. This offers the additional advantage of being able to detect potential iodine deficiency “hot spots” using the birth hospital postcode as a locator.
Received 11 May 2010, accepted 28 July 2010
- Ashequr Rahman1
- Gayle S Savige1
- Nicholas J Deacon2
- Ivan Francis3
- Janice E Chesters1
- 1 Monash University Department of Rural and Indigenous Health, Moe, VIC.
- 2 School of Applied Science and Engineering, Monash University Gippsland Campus, Churchill, VIC.
- 3 Victorian Clinical Genetics Services, Melbourne, VIC.
We thank Mark Oakley Browne for his support in gaining Ashequr Rahman a Fellowship from SAS Institute Australia Pty Ltd for software support.
None identified.
- 1. Bernal J, Nunez J. Thyroid hormones and brain development. Eur J Endocrinol 1995; 133: 390-398.
- 2. Koibuchi N, Chin WW. Thyroid hormone action and brain development. Trends Endocrinol Metab 2000; 11: 123-128.
- 3. Chan S, Kilby MD. Thyroid hormone and central nervous system development. J Endocrinol 2000; 165: 1-8.
- 4. World Health Organization, United Nations Children’s Fund, and International Council for the Control of Iodine Deficiency Disorders. Assessment of iodine deficiency disorders and monitoring their elimination. A guide for programme managers. 3rd ed. Geneva, Switzerland: WHO, 2007.
- 5. World Health Organization. Iodine status worldwide: a global database on iodine deficiency. Geneva, Switzerland: WHO, 2004.
- 6. Li M, Eastman CJ, Waite KV, et al. Are Australian children iodine deficient? Results of the Australian National Iodine Nutrition Study. Med J Aust 2006; 184: 165-169. <MJA full text>
- 7. Craig PE, Cooper-Stanbury M. Fortification monitoring: tracking mandatory folic acid and iodine fortification in Australia and New Zealand [abstract]. Australas Med J 2010; 1, 1: 74-96. http://www.amj.net.au/index.php?journal=AMJ& page=article&op=viewFile&path[]=207&path[]=485 (accessed Aug 2010).
- 8. Delange F. Screening for congenital hypothyroidism used as an indicator of the degree of iodine deficiency and of its control. Thyroid 1998; 8: 1185-1192.
- 9. Zimmerman M, Aeberli I, Torresani T, Bürgi H. Increasing the iodine concentration in the Swiss iodized salt program markedly improved iodine status in pregnant women and children: a 5-y prospective national study. Am J Clin Nutr 2005; 82: 388-392.
- 10. Tylek-Lemanska D, Rybakowa M, Kumorowicz-Kopiec M, et al. Iodine deficiency disorders incidence in neonates based on the experience with mass screening for congenital hypothyroidism in southeast Poland in the years 1985–2000. J Endocrinol Invest 2003; 26 (2 Suppl) : 32-38.
- 11. Stoeva I, Timcheva T, Antova R. The neonatal TSH screening as monitor of the iodine supplementation program in Bulgaria 1997–2007 [abstract]. Hormone Res 2008; 70 Suppl 1: 242-243.
- 12. Charoensiriwatana W, Srijantr P, Janejai N, Hasan S. Application of geographic information system in TSH neonatal screening for monitoring of iodine deficiency areas in Thailand. Southeast Asian J Trop Med Public Health 2008; 39: 362-367.
- 13. Zimmermann MB. Iodine deficiency. Endocr Rev 2009; 30: 376-408.
- 14. McDonnell CM, Harris M, Zacharin MR. Iodine deficiency and goitre in schoolchildren in Melbourne, 2001. Med J Aust 2003; 178: 159-162. <MJA full text>
- 15. Victorian Clinical Genetics Services. Newborn screening. http://www.vcgspathology.com.au/sections/NewbornScreening/?docid=b4d824a1-5c71-420a-b056-992e00efe8f3 (accessed Aug 2010).
- 16. Stone C, Lester R. Beyond the crystal ball: the epidemiology of some genetic conditions in Victoria. Melbourne: Public Health Group, Department of Human Services, 2002.
- 17. The Consultative Council on Obstetric and Paediatric Mortality and Morbidity (CCOPMM). Annual report for the year 2007. Hospital and Health Services Performance Division. Victorian Government Department of Health. Melbourne, Victoria, Australia. April 2010. http://www.health.vic.gov.au/ccopmm/downloads/ccopmm_annrep07.pdf (accessed Sep 2010).
- 18. McElduff A, McElduff P, Gunton JE, et al. Neonatal thyroid-stimulating hormone concentrations in northern Sydney: further indications of mild iodine deficiency? Med J Aust 2002; 176: 317-320. <MJA full text>
- 19. McElduff A, McElduff P, Wiley V, et al. Neonatal thyrotropin as measured in a congenital hypothyroidism screening program: influence of the mode of delivery. J Clin Endocrinol Metab 2005; 90: 6361-6363.
- 20. Travers CA, Guttikonda K, Norton CA, et al. Iodine status in pregnant women and their newborns: are our babies at risk of iodine deficiency? Med J Aust 2006; 184: 617-620. <MJA full text>
- 21. Li M, Eastman CJ. Neonatal TSH screening: is it a sensitive and reliable tool for monitoring iodine status in populations? Best Pract Res Clin Endocrinol Metab 2010; 24: 63-75.
- 22. American Academy of Pediatrics, Rose SR; Section on Endocrinology and Committee on Genetics, American Thyroid Association, Brown RS; Public Health Committee, Lawson Wilkins Pediatric Endocrine Society, Foley T, Kaplowitz PB, Kaye CI, et al. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics 2006; 117: 2290-2303.
- 23. Revised guidelines for neonatal screening programmes for primary congenital hypothyroidism. Hormone Res 1999; 52: 49-52.
- 24. American Academy of Pediatrics Section on Endocrinology and Committee on Genetics and American Thyroid Association Committee on Public Health. Newborn screening for congenital hypothyroidism: recommended guidelines. Pediatrics 1993; 91: 1203-1209.





Abstract
Objective: To use neonatal thyroid-stimulating hormone (TSH) concentration data to measure the iodine status of the population of the Australian state of Victoria.
Design, participants and setting: Retrospective analysis of the results of 368 552 neonatal heel-prick blood tests for TSH concentration in Victoria in the years 2001–2006.
Main outcome measures: Iodine deficiency as indicated by a mean percentage of neonatal TSH concentrations > 5 mIU/L of over 3% in accordance with World Health Organization, United Nations Children’s Fund and International Council for the Control of Iodine Deficiency Disorder criteria; comparison of findings for the nine Department of Human Services health regions in Victoria.
Results: The mean percentage of neonatal TSH concentrations > 5 mIU/L ranged from 4.07% in 2001 to 9.65% in 2006, and this increase was statistically significant (P < 0.001). The populations of all nine Victorian health regions showed increasing iodine deficiency over the study period. Metropolitan populations had higher iodine deficiency than non-metropolitan populations, and this difference was also statistically significant (P < 0.05). These results are consistent with urinary iodine excretion research in Victoria.
Conclusions: The high percentage of elevated TSH concentrations among newborns is of concern and requires ongoing monitoring. Neonatal TSH assay is part of routine screening in Australia, and thus offers an effective and economical method of monitoring population iodine status.