While many viruses that infect humans in Australia are found worldwide, some are unique to our country or region. Some of these are classed as "emerging", including:
Hendra and Menagle viruses, which were identified for the first time in Australia, in 1994 and 1997, respectively,1,2 and have not been found elsewhere. Another new agent, Nipah virus, has been found in nearby Malaysia and Singapore, but has not yet appeared in Australia.
Australian bat lyssavirus, which is also newly described and unique to Australia, but is closely related to lyssaviruses found elsewhere.
Murray Valley encephalitis and Kunjin viruses, which have been found predominantly in Australia for many years but are now causing an increasing incidence of infection.
Japanese encephalitis virus, which is widespread in many of our South-East Asian neighbours and was recently encountered in Australia for the first time.
The clinical and epidemiological features of these viruses are summarised in Box 1. Infection with these viruses has no specific treatment; management comprises supportive measures.
Hendra virus infection has been identified in three people. In Brisbane, in 1994, a 49-year-old horse trainer died after a fulminating septic pneumonic illness, while a 40-year-old worker at his stable survived an influenza-like illness. Eighteen horses in the same stable developed a pneumonic illness, and fourteen died. Serological evidence of infection was demonstrated in three other horses which were asymptomatic.1,3 A year later, a 36-year-old farmer died as a result of Hendra virus encephalitis in Mackay, Queensland.4 Two of his horses were subsequently shown to have died of Hendra virus infection 13 months earlier.
Subsequent seroepidemiological testing for Hendra virus in a large number of human contacts of these three people was completely negative.5 Seroepidemiological studies of more than 2000 horses and more than 5000 samples from 46 other animal species in Queensland also failed to identify a single Hendra virus infection.6 However, antibody to Hendra virus was found in 20 of 224 serum samples from four species of fruit bats from as far north as Madang, Papua New Guinea, and as far south as Melbourne.7 Fruit bats (Box 2) now seem a likely source of Hendra virus, although they appear to be asymptomatically infected.
Menangle virus was isolated from stillborn piglets in New South Wales in 1997, and serological evidence of infection was found in pigs, humans and fruit bats closely associated with the piggery. Two piggery workers developed an influenza-like illness with a spotty, red, non-pruritic rash.2 No further cases have been identified. The wider prevalence of Menangle virus remains to be determined.
An outbreak of encephalitis involving more than 250 pig farmers occurred in northern Malaysia in 1999, with a 40% case-fatality rate.8 At about the same time, an outbreak occurred in Singapore involving 11 abattoir workers who had fever with pneumonia or encephalitis; one died.9 Both outbreaks were shown to be caused by Nipah virus (named after the town of first recognition in Malaysia). Ribavirin therapy was used in many patients without obvious therapeutic benefit.8 More research is required to define the epidemiology of this virus, although pigs seem to be involved asymptomatically as intermediate hosts. Nipah virus infection has not yet been reported in Australia.
Infection with Australian bat lyssavirus (ABL) has been identified in two people. In 1996, a 39-year-old woman presented with weakness of the arm and subsequent progressive neurological deterioration with bulbar palsy.10 She had been exposed to a number of animals, including bats. Her condition deteriorated, and she died on Day 21 of the illness.10 In 1998, a 37-year-old woman died of a rabies-like illness, 27 months after being bitten by an Australian flying fox (fruit bat).11
Four species of Australian flying foxes and one species of insectivorous bat have been shown to be infected with ABL.12 Infected bats were distributed from Darwin to Melbourne. The proportion of bats in the wild that are infected is not known.
ABL is closely related genetically to rabies virus. Both the clinical manifestations and pathological changes of ABL infection in the human cases were very similar to those of rabies, with meningoencephalomyelitis and neuronal intracytoplasmic inclusions. Rabies vaccine and immunoglobulin offer significant protection against ABL.
ABL infection should be suspected in patients who present with a progressive neurological condition and history of exposure to bats (Box 1). The diagnosis is made by polymerase chain reaction tests for ABL in cerebrospinal fluid or serum antibody tests. Examination of bat brain tissue can also be helpful but is rarely possible.
Once the disease develops, there is no specific treatment. Post-exposure prophylaxis should be given after a bite, scratch or significant salivary exposure from a bat in Australia (Boxes 2 and 4). Prophylaxis is not indicated after exposure to bat urine or faeces. As long incubation periods (over a year) have been anecdotally described for lyssavirus infection, there is no cut-off period of infectivity after exposure. However, it is suggested that rabies immunoglobulin can be omitted if exposure occurred more than a year earlier.13
Pre-exposure prophylaxis with three doses of rabies vaccine, at Days 0, 7 and 28, is recommended for expatriates and travellers spending more than one month in rural parts of endemic areas (advice on high-risk countries is found on the World Health Organization website <www.who.int/>, and on rabies-free countries in the Australian immunisation handbook13). In Australia, pre-exposure prophylaxis is recommended for people at risk of bites or scratches from bats (eg, bat handlers, veterinarians, wildlife officers and others who are liable to come into direct contact with bats).13
Murray Valley encephalitis (MVE) virus and the closely related Kunjin virus are flaviviruses that cause encephalitis, although Kunjin virus more commonly produces a non-encephalitic illness with polyarthralgia. MVE virus is endemic in avian species and is found in humans in northern Western Australia, the Northern Territory and Queensland.14 The last MVE epidemic occurred in 1974, with the Murray Valley region as epicentre.15 Kunjin virus occurs over a much wider area, including most of tropical Australia.
There is some evidence that infection with these viruses is increasing in incidence. Nine cases of encephalitis caused by MVE or Kunjin virus and five non-encephalitic cases were identified in Western Australia between March and July, 2000.16 Recently, seroconversion in sentinel chicken flocks was documented in western NSW for the first time since serological testing began over 20 years ago (Dr Dominic Dwyer, Institute of Clinical Pathology and Medical Research, Westmead Hospital, NSW, personal communication). In northern Australia, cases occur predominantly between February and July, corresponding to the end of the monsoon season, when the mosquito vector (Culex annulirostris) proliferates in flooded environments.
Although seroprevalence in enzootic areas ranges from 39% to 46%,17 clinical illness is rare. The clinical and epidemiological features of MVE in the Northern Territory, where the virus is endemic, were described for 16 of 18 identified cases between 1987 to 1996. Most cases were in children. Fever was universal, and some cases had a variable prodrome, which included diarrhoea, rash and cough. Seizures were common in children, while adults more commonly presented with headache, dysphasia, memory impairment, confusion and tremor. Several distinct clinical patterns were noted, including relentless progression to death, a poliomyelitis-like illness, cranial nerve or brainstem involvement with tremor, and a non-specific encephalitic illness. Mortality has been estimated at about 20%, with residual neurological impairment in about half the survivors.18
Kunjin virus infection presents with an acute febrile illness and polyarthralgia. The major differential diagnosis is infection with Ross River or Barmah Forest virus. Encephalitis occurs rarely.
Japanese encephalitis (JE) virus is another flavivirus that is considered rare in Australia. However, in 1995, JE was documented in the Torres Strait Islands, with three cases, two of which were fatal.19 Seroepidemiological studies revealed relatively widespread infection in humans and pigs on at least nine islands. Subsequently, in 1998, a human case was recognised in mainland Australia, in a fisherman from the Mitchell River on Cape York.20
The global importance of JE is significant, with about 50 000 cases and 15 000 deaths a year worldwide.21 It occurs across eastern and southern Asia and the Pacific rim (Box 5). It is transmitted between animals by Culex mosquitoes, with an enzootic cycle involving wild and domestic birds and animals, particularly pigs. Humans are infected as accidental hosts, and, as human viraemia is usually brief and of low concentration, rarely transmit the virus. The ratio of symptomatic to asymptomatic infection is estimated to be between 1: 25 and 1: 1000.22 Two epidemiological patterns are recognised: 23 epidemics occur during the summer in northern areas (eg, northern Vietnam, northern Thailand, Korea, Japan, Taiwan, China, Nepal and northern India), whereas the disease tends to be endemic throughout the year in southern areas (southern Vietnam, southern Thailand, Indonesia, Malaysia, the Philippines, Sri Lanka and southern India).
JE has a short prodrome which may include coryza, diarrhoea, and rigors.21 Headache, vomiting, obtundation and seizures may ensue, with some individuals presenting with personality change or abnormal behaviour. Seizures are common, particularly in children, and extrapyramidal features, such as tremor, hypertonia, cogwheel rigidity, choreoathetosis, opsoclonus, myoclonus and lip smacking, are suggestive of JE.21 Upper motor neurone facial nerve palsies and opisthotonos or a clinical syndrome of acute flaccid paralysis may occur.24 Overall mortality is about 30%, with residual neurological damage in about half the survivors.
Diagnosis is usually serological, with enzyme immunoassay IgM and IgG capture assays useful for testing serum and cerebrospinal fluid (case history, Box 6).
No specific therapeutic agent has proven beneficial, but there is early suggestion of improvement with interferon-α.
A formalin-inactivated vaccine is available for prevention and is recommended for:
residents of endemic areas;
laboratory workers potentially exposed to the virus; and
travellers spending 30 days or longer in endemic areas, particularly if they intend to spend a lot of time outdoors in rural areas during the wet season.13
The vaccine is administered in a series of three doses, at 0, 7 and 30 days, with a booster recommended at one year. Tenderness, redness and swelling occur in up to 20% of recipients, and fever, headache, malaise and chills in about 10%. The combination of itching, urticaria, and occasionally angio-oedema of the face, which can be severe, has been recognised since 1989, with an incidence estimated at 2–10 per 1000 vaccine doses, and higher in those with a history of urticaria.
Infection with these seven viruses may present similarly — an acute febrile illness and encephalitis are common. ABL may present similarly to rabies. The epidemiological pattern is an important clue to diagnosis, and questions about patients' residence, travel and contacts are vital.
The spectrum of disease and geographical distribution of viral infections in Australia continue to evolve, providing an ongoing challenge for clinicians and researchers. The increased ease of travel has reduced the remoteness of Australasia. Recent experience has shown that new infectious diseases can emerge from both within and outside Australia, and further new viral infections can be expected in the future.
Evidence-based recommendations
Rabies vaccine and immunoglobulin offer protection against infection with Australian bat lyssavirus and should be given after a bite or scratch from a bat in Australia13 (E4).
Vaccine against Japanese encephalitis should be recommended for people who intend to live in an endemic area for over 30 days13 (E4).
1: Clinical and epidemiological features of emerging viral infections in Australia
Virus |
Epidemiological clues |
Clinical features |
Key investigations |
Prevention |
|||||||
Hendra |
Contact with fruit bats or horses in eastern Australia or Papua New Guinea |
Flu-like illness, pneumonia, encephalitis |
Hendra virus antibodies in serum |
Avoid contact with fruit bats or sick horses in endemic areas |
|||||||
Menangle |
Contact with stillborn piglets and bats in New South Wales |
Flu-like illness, rash |
Menangle virus antibodies in serum |
Avoid contact with piglets or fruit bats in endemic areas |
|||||||
Nipah |
Contact with pigs in Malaysia or Singapore |
Flu-like illness, pneumonia, encephalitis |
Nipah virus antibodies in serum |
Avoid contact with pigs in endemic areas |
|||||||
Australian bat lyssavirus (ABL) |
Contact with bats in Australia |
Similar to rabies: acute, progressive neurological disorder |
Polymerase chain reaction tests of cerebrospinal fluid ±
lyssavirus antibodies in serum
|
Avoid contact with bats; immunoprophylaxis (Box 3) |
|||||||
Murray Valley encephalitis (MVE) |
Residence in northern Australia or Papua New Guinea, especially during wet season (February to July) |
Acute febrile illness, encephalitis |
MVE antibodies in serum |
Mosquito avoidance measures |
|||||||
Kunjin |
Residence in Australia (particularly northern Australia), especially during wet season (February to July) |
Acute febrile illness, polyarthralgia, and (rarely) encephalitis |
Kunjin antibodies in serum |
Mosquito avoidance measures |
|||||||
Japanese encephalitis (JE) |
Residence in South-East or East Asia, Indian subcontinent or Torres Strait; exposure to domestic birds and animals, especially pigs |
Flu-like illness, encephalitis |
JE antibodies in serum or cerebrospinal fluid |
Mosquito avoidance measures; JE vaccine if intend to travel to endemic areas for longer than 30 days |
|||||||
3: Post-exposure treatment for rabies and Australian bat lyssavirus in non-immune people*
Immediate local treatment (Day 0)
Wound cleansing with soap and water or antiseptic solution is vital; debridement if indicated.
Rabies vaccine
Immediate (Day 0) administration of 1.0 mL intramuscularly, followed by 1.0 mL on Days 3, 7, 14 and 28.
Rabies immunoglobulin
20 IU/kg of immunoglobulin (150 IU/mL) should be given no later than seven days after the first dose of rabies vaccine; as much as possible should be infiltrated around the wound site, with the remainder given intramuscularly.
* In people who have received pre-exposure prophylaxis, a modified post-exposure rabies vaccine regimen is recommended (ie, doses at Days 0 and 3). Rabies immunoglobulin is not recommended.
5: Distribution of Japanese encephalitis, 1970–1998
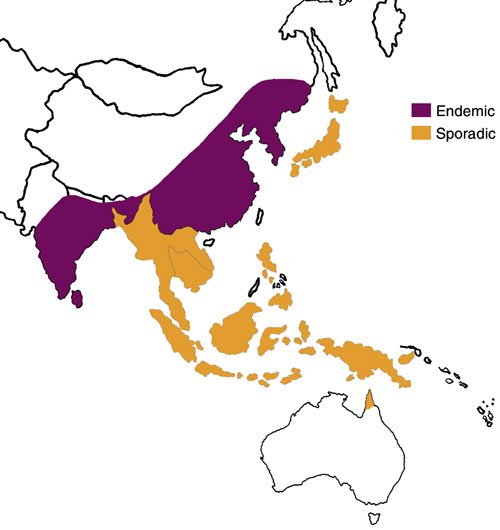
South-East Asia and Australia, showing areas where Japanese encephalitis is endemic and sporadic. (Adapted from Centers for Disease Control website <www.cdc.gov/ncidod/dvbid/jencephalitis/map.htm> and <www.cdc.gov/travel/jenceph.htm>)
6: Case history — Japanese encephalitis
Presentation: A 65-year-old man presented with a three-day history of fever, rigors, headache and progressive confusion. He had recently returned on holiday from north Vietnam, where he had lived for several years.
Examination: He was disoriented, could not remember where he lived, had difficulty recognising his children and was incontinent of urine on several occasions. On examination, he had myoclonus of his upper limbs, with hypertonicity and hyperreflexia of all limbs, and extensor plantar responses. The rest of his physical examination gave normal results. Over the following 24 hours, he developed tonic–clonic seizures and choreoathetotic and lip-smacking movements.
Investigations: Computed tomography and magnetic resonance imaging of the head showed no abnormalities. Cerebrospinal fluid (CSF) was clear, with raised concentrations of mononuclear cells (100 x 106/L; reference range [RR], < 5 x 106/L), and protein (1.2 g/L; RR, 0.15–0.45 g/L). Japanese encephalitis was diagnosed on the basis of enzyme immunoassay IgM and IgG capture assays of serum and CSF.
Management and course: The patient was treated with intravenous fluids, anticonvulsants and empirical aciclovir. Over the following week, he had progressive neurological deterioration culminating in loss of consciousness and death. No autopsy was performed.
The possibility of Japanese encephalitis was suggested by:
a prodromal febrile illness;
rapidly progressive encephalitis;
residence in, or travel to, an endemic area; and
extrapyramidal features.
- 1. Selvey LA, Wells RM, McCormack JG, et al. Infection of humans and horses by a newly described morbillivirus. Med J Aust 1995; 162: 642-645.
- 2. Chant K, Chan R, Smith M, et al. Probable human infection with a newly described virus in the family Paramyxoviridae. The NSW Expert Group. Emerg Infect Dis 1998; 4: 273-275.
- 3. Paterson DL, Murray PK, McCormack JG. Zoonotic disease in Australia caused by a novel member of the paramyxoviridae. Clin Infect Dis 1998; 27: 112-118.
- 4. O'Sullivan JD, Allworth AM, Paterson DL, et al. Fatal encephalitis due to novel Paramyxovirus transmitted from horses. Lancet 1997; 349: 93-95.
- 5. McCormack JG, Allworth AM, Selvey LA, Selleck PW. Transmissibility from horses to humans of a novel paramyxovirus, equine morbillivirus (EMV). J Infect 1999; 38: 22-23.
- 6. Ward MP, Black PF, Childs AJ, et al. Negative findings from serological studies of equine Morbillivirus in the Queensland horse population. Aust Vet J 1996; 74: 241-243.
- 7. Young PL, Halpin K, Selleck PW, et al. Serological evidence for the presence in Pteropus bats of a paramyxovirus related to equine morbillivirus. Emerg Infect Dis 1996; 2: 239-240.
- 8. Goh KJ, Tan CT, Chow NK. Clinical features of Nipah virus among pig farmers in Malaysia. N Engl J Med 2000; 342: 1229-1235.
- 9. Paton NI, Leo YS, Zaki SR, et al. Outbreak of Nipah virus infection among abattoir workers in Singapore. Lancet 1999; 354: 1253-1256.
- 10. Allworth A, Murray K, Morgan J. A human case of encephalitis due to a Lyssavirus recently identified in fruit bats. Commun Dis Intell 1996; 20: 504.
- 11. Hanna JN, Carney IK, Smith GK, et al. Australian bat Lyssavirus infection: a second human case, with a long incubation period. Med J Aust 2000; 172: 597-599. <eMJA full text>
- 12. Mackenzie JS, Chua KB, Daniels PW, et al. Emerging viral diseases of southeast Asia and the western Pacific. Emerg Infect Dis 2001; 7 suppl 3: 497-504.
- 13. National Health and Medical Research Council. The Australian immunisation handbook. 7th ed. Canberra: AGPS, 2000. Was also available at http://www.health.gov.au:80/HFS/publth/strateg/communic/lyshlth.htm - updated version available at http://www.immunise.health.gov.au/handbook.htm
- 14. Mackenzie JS, Broom AK, Hall RA. Arboviruses in the Australian region, 1990 to 1998. Commun Dis Intell 1998; 22: 93-100.
- 15. Bennett NM. Murray Valley encephalitis, 1974: clinical features. Med J Aust 1976; 2: 446-450.
- 16. Cordova SP, Smith DW, Broom AK, et al. Murray Valley encephalitis in Western Australia in 2000, with evidence of southerly spread. Commun Dis Intell 2000; 24: 368-372.
- 17. Holland J, Smith DW, Broom AK, et al. A comparison of seroprevalence of arboviral infections between three Northern Territory regions. Aust Microbiol 1994; 15: 105.
- 18. Burrow JN, Whelan PI, Kilburn CJ, et al. Australian encephalitis in the Northern Territory: Clinical and epidemiological features, 1987-1996. Aust N Z J Med 1998; 28: 590-596.
- 19. Hanna JN, Ritchie SA, Phillips DA, et al. An outbreak of Japanese encephalitis in the Torres Strait, Australia, 1995. Med J Aust 1996; 165: 256-260. <eMJA full text>
- 20. Hanna JN, Ritchie SA, Phillips DA, et al. Japanese encephalitis in north Queensland, Australia, 1998. Med J Aust 1999; 170: 533-536.
- 21. Solomon T, Dung NM, Kneen R, et al. Japanese encephalitis. J Neurol Neurosurg Psychiatry 2000; 68: 405-415.
- 22. Huang CH. Studies of Japanese encephalitis in China. Adv Virus Res 1982; 27: 71-101.
- 23. Vaughn DW, Hoke CH Jr. The epidemiology of Japanese encephalitis: prospects for prevention. Epidemiol Rev 1992; 14: 197-221.
- 24. Solomon T, Kneen R, Dung NM, et al. Poliomyelitis-like illness due to Japanese encephalitis virus. Lancet 1998; 351: 1094-1097.






Abstract
Hendra virus infection should be suspected in someone with close association with horses or bats who presents acutely with pneumonia or encephalitis (potentially after a prolonged incubation period).
Australian bat lyssavirus infection should be suspected in a patient with a progressive neurological illness and a history of exposure to a bat.
Rabies vaccine and immunoglobulin should be strongly considered after a bite, scratch or mucous membrane exposure to a bat.
Japanese encephalitis vaccine should be considered for people intending to reside in or visit endemic areas of southern or eastern Asia for more than 30 days.