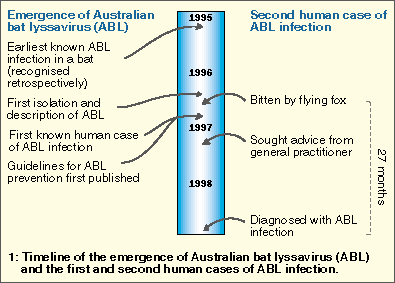
In December 1998, a 37-year-old Queensland woman died from a rabies-like illness, 27 months after being bitten by a flying fox (fruit bat). Molecular techniques enabled diagnosis of infection with Australian bat lyssavirus (ABL), the second human case to be recognised and the first to be acquired from a flying fox. It must be assumed that any bat in Australia could transmit ABL; anyone bitten or scratched by a bat should immediately wash the wounds thoroughly with soap and water and promptly seek medical advice.
The Australian bat lyssavirus (ABL) was first recognised in June 1996.1 It was subsequently shown not only to belong to a new genotype within the Lyssavirus genus, but also to be more closely related to classic rabies virus than any of the other five genotypes of lyssavirus.2
The first recognised human infection with ABL was in November 19961,3 (Box 1). The patient died from a rabies-like illness 20 days after first becoming unwell. She had apparently been bitten by a yellow-bellied sheathtail bat (Saccolaimus flaviventris; an insectivorous bat) about 4.5 weeks before onset of the illness (R Taylor, Public Health Physician, Rockhampton, QLD, personal communication). We describe here the features of the second recognised human infection with ABL, which had a much longer incubation period and was transmitted by a flying fox (fruit bat; Pteropus sp.).
Clinical record
A 37-year-old woman was admitted to Mackay Base Hospital in late November 1998 with a five-day history of fever, vomiting, anorexia, pain about the left shoulder girdle, paraesthesiae about the dorsum of her left hand and sore throat with difficulty swallowing.
On examination she was acutely ill but well oriented. She was unable to fully open her mouth, was drooling saliva and had difficulty speaking. She was febrile (38 degrees C) but normotensive. Muscle tone was increased, and examination occasionally provoked painful spasms. Examination of the throat provoked spasmodic attempts to swallow. Apart from neutrophilia (12.0 x 109/L; reference range, 2.0-8.0 x 109/L), routine haematological and biochemical tests gave normal results.
Twelve hours later her condition had deteriorated considerably, with increased agitation, dysphagia and dysphonia, and the muscular spasms had become more frequent and severe. She was paralysed and ventilated. At about this time, a history of a bat bite was elicited, and a diagnosis of ABL infection was considered. Cerebrospinal fluid (CSF), serum and saliva were submitted for testing.
An attempt on Day 2 of hospitalisation to cease artificial ventilation was unsuccessful; when the sedative dose was reduced, she was no longer able to communicate and did not appear to understand verbal commands. Thereafter, she remained ventilator-dependent; whenever the dose of muscle relaxants was reduced, purposeless movements, such as facial grimacing and rolling eye movements, and muscular spasms, such as arching of the back, became evident. Another prominent feature of the illness was marked fluctuations of body temperature and blood pressure.
On Day 4 of hospitalisation, the reference laboratory reported that a polymerase chain reaction (PCR) had detected what appeared to be a specific ABL product in the saliva. Nursing and medical staff were informed of the probable diagnosis of ABL infection, and appropriate precautions were implemented.4 The diagnosis of ABL infection was confirmed four days later.
On Day 14 of hospitalisation, the patient ceased spontaneous movements and respiratory effort. Ventilator support was withdrawn; she died 19 days after onset of the illness. Post-exposure treatment (PET) was provided to seven healthcare workers because of possible percutaneous or mucous membrane exposure to the patient's saliva.4,5
The patient had attended an evening barbecue in late August 1996, 27 months before onset of the illness (Box 1). At the function, a flying fox had suddenly landed on the back of a young child. In the course of removing it, the patient was bitten at the base of her fifth left finger.
Two days later, she presented to her general practitioner and was given tetanus toxoid and appropriate antibiotics. Six months later, in early March, she returned to the GP, asking about a blood test for the "bat virus". She was advised that she should instead receive PET because of a potential exposure to ABL, but decided against this.
As soon as the diagnosis of ABL infection was confirmed, PET was administered to the child and to four other people exposed to the flying fox at the barbecue.
Diagnostic studies
No antibodies to Japanese encephalitis (JE), Murray Valley
encephalitis, Kunjin or rabies viruses were detected by enzyme
immunoassay tests of serum and CSF collected on Day 2 of
hospitalisation. Attempts were made to culture virus by inoculating
serum, CSF and saliva onto monolayers of C636, BHK-21, Vero and mouse
neuroblastoma cells, but no viruses were isolated.
Serum, CSF and saliva were examined for RNA of JE virus, Hendra virus (formerly known as equine morbillivirus) by in-house reverse transcriptase PCR, and for RNA of ABL by heminested reverse transcriptase PCR.6 No viral RNA was detected in serum or CSF, and neither JE nor Hendra virus RNA was detected in saliva. However, the heminested PCR for ABL in saliva produced amplicons of the expected size6 in both first- and second-round reactions (600 and 586 base pairs, respectively). Nucleotide sequencing showed that the amplicon was a lyssavirus-specific product that differed from any other ABL held at the reference laboratory.
Concurrently, products from the amplification were separated electrophoretically, transferred onto a nylon membrane7 and hybridised with a digoxigenin-labelled ABL-specific probe. The probe hybridised with the control virus and with amplicons generated from the saliva, indicating that the amplicons were indeed lyssavirus-specific products.
Postmortem studies
Light microscopy revealed widespread and severe encephalitis
affecting all parts of the brain other than the cerebellum.
Inflammation and necrosis were particularly severe in the brainstem
and hippocampi, where most neurones had either completely
disappeared or were necrotic. There was perivascular cuffing by
lymphocytes and diffuse infiltration of grey matter neuropile by
microglia and lipid-laden macrophages. Occasional neurones showed
neuronophagia. A few cytoplasmic inclusion bodies were seen,
particularly in the hypothalamus.
Light microscopy also revealed diffuse pancarditis with focal myocyte destruction and infiltration of epicardial nerve branches with mononuclear cells. There was no evidence of viral inclusions in acinar or ductal epithelial cells of the parotid and submandibular glands, but nerve bundles in each were infiltrated by mononuclear cells.
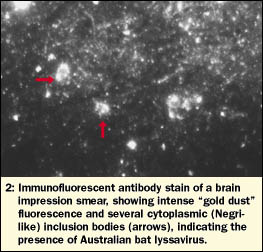
Spinal cord, brainstem, cerebellum, midbrain and both cerebral hemispheres were examined by immunofluorescent antibody staining. Intense fluorescence was observed in all impression smears, indicating the widespread presence of ABL (Box 2). RNA extracted from these brain samples, as well as salivary and adrenal glands, was tested for ABL by heminested PCR; all samples were strongly positive.
Portions of each tissue section were cultured with mouse neuroblastoma cells. PCR indicated successful virus isolation from brain and spinal cord after the first blind passage (Day 7 after inoculation), while specific immunofluorescent antibody staining of cells, indicating presence of ABL, was evident after the second blind passage (Day 14). Sequencing of the PCR product from the cell cultures confirmed that the isolate was the flying-fox variant of ABL.
Public health responses
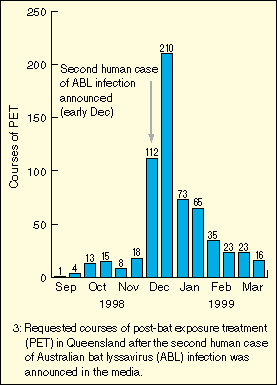
As soon as the patient's diagnosis was announced in the media,
requests for PET increased markedly throughout Queensland. Many
requests were for exposures that had occurred many months, sometimes
years, previously. Five hundred and eighteen courses were requested
between December 1998 and February 1999 (inclusive), compared with
24 courses in the same three months the previous year, and 59 courses in
the preceding three months (Box 3).
Discussion
The clinical presentation, duration and course of the patient's illness were virtually indistinguishable from those seen in rabies. A short, non-specific prodrome was followed by inexorable progression through distinct stages, culminating, after a relatively short illness, in coma and death. Pain and paraesthesiae about the site of the bite and signs of autonomic instability, such as hypersalivation and labile blood pressure, are also commonly seen in rabies.8
The histological features in the brain were similar to those seen in rabies, including the pathognomonic Negri-like inclusion bodies.8 Findings similar to those seen in the heart and salivary glands have been described in patients who died of rabies.9
Monoclonal antibody and molecular sequencing studies have shown distinct variants of rabies virus, each associated with a dominant mammalian reservoir.10 The first reported patient with ABL infection was infected with the virus variant associated with yellow-bellied sheathtail bats (A Gould, Senior Principal Research Officer, Australian Animal Health Laboratory, Geelong, Vic, personal communication), and, as expected, our patient was infected with the flying-fox variant. ABL has been found in all four Australian flying fox species, and, to date, all ABL-infected bats have been either unwell or dead at the time of collection.11
An extraordinary feature of the patient's illness was the very long (27 months) incubation period. The usual incubation period for rabies is 20-90 days, and 95% of cases occur within a year of exposure.8 Although rare, prolonged incubation periods have been reported,12,13 but the reason for the prolongation has not been established.
Even if the patient had accepted the recommended PET, it is uncertain whether the illness would have been prevented. This is because the guidelines at the time recommended vaccine only, without rabies immunoglobulin, as treatment after a bat exposure more than three months previously.5 Rabies that occurred because PET was either delayed or did not include rabies immunoglobulin has been reported.14 The guidelines were subsequently changed to recommend rabies immunoglobulin for all bat exposures,15 but most public health authorities do not include this immunoglobulin if the potential exposure to ABL was more than 12 months previously.
Media reporting of the patient's diagnosis was initially restrained but changed on her death, with some reports becoming blatantly alarmist. This media attention and the inherent concern about rabies contributed to intense public demand for PET from Queensland public health units. Although the cost of this PET was considerable, it was predominantly for "catch-up" treatments for those with historical exposures and therefore probably represents a "one-off" expense.
This case reminds that ABL infection, although rare, is lethal. Any bat in Australia must be assumed to have the potential to transmit the virus, and members of the public should therefore avoid handling bats. Anyone either bitten or scratched by a bat should immediately wash the wounds thoroughly with soap and water and promptly seek medical advice, regardless of the site or severity of the exposure.
Acknowledgements
Many people were involved in the management of the patient and in the public health responses. We wish to thank the nursing staff of the Intensive Care Unit, Mackay Base Hospital, and the Public Health Nurses, in particular Mrs Dorothy Symons, of the Tropical Public Health Unit Network. We also thank Ms Judy Northill and Mr Alan Westacott (Queensland Health Scientific Services) and Mr David Gould (Communicable Diseases Unit, Queensland Health).
References
- Hooper PT, Lunt RA, Gould AR, et al. A new lyssavirus -- the first
endemic rabies-related virus recognised in Australia. Bull Inst
Pasteur 1997; 95: 209-218.
- Gould AR, Hyatt AD, Lunt R, et al. Characterisation of a novel lyssavirus isolated from Pteropid bats in Australia. Virus Res 1998; 54: 165-187.
- Samaratunga H, Searle JW, Hudson N. Non-rabies Lyssavirus human encephalitis from fruit bats: Australian bat Lyssavirus (pteropid Lyssavirus) infection. Neuropathol Appl Neurobiol 1998; 24: 331-335.
- Helmick CG, Tauxe RV, Vernon AA. Is there a risk to contacts of patients with rabies? Rev Infect Dis 1987; 9: 511-518.
- Lyssavirus Expert Group. Prevention of human lyssavirus infection. Comm Dis Intell 1996; 20: 505-507.
- Heaton PR, Johnstone P, McElhinney LM, et al. Heminested PCR assay for detection of six genotypes of rabies and rabies-related viruses. J Clin Microbiol 1997; 35: 2762-2766.
- Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 1975; 98: 503-517.
- Fishbein DB, Bernard KW. Rabies virus. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York: Churchill Livingstone, 1995: 1527-1543.
- Jackson AC, Ye H, Phelan CC, et al. Extraneural organ involvement in human rabies. Lab Invest 1999; 79: 945-951.
- Krebs JW, Smith JS, Rupprecht CE, Childs JE. Rabies surveillance in the United States during 1997. J Am Vet Med Assoc 1998; 213: 1713-1728.
- Barrett J, Young P, Field H, et al. Australian bat lyssavirus in Queensland. Abstracts of the Forty-eighth Annual Wildlife Disease Association Conference; 1999 Aug 8-12; Athens (Ga), abstract no 33.
- Smith JS, Fishbein DB, Rupprecht CE, Clark K. Unexplained rabies in three immigrants in the United States: a virologic investigation. N Engl Med J 1991; 324: 205-211.
- Grattan-Smith PJ, O'Regan WJ, Ellis PSJ, et al. Rabies, a second Australian case, with a long incubation period. Med J Aust 1992; 156: 651-654.
- Wilde H, Choomkasien P, Hemachudha T, et al. Failure of rabies postexposure treatment in Thailand. Vaccine 1989; 7: 49-52. Rabies and Australian bat lyssavirus: recommendations for pre- and post-exposure vaccination. Comm Dis Intell 1998; 22: 153.
(Received 17 Dec 1999, accepted 21 Mar 2000)
Authors' details
Tropical Public Health Unit, Queensland Health, Cairns, QLD.Jeffrey N Hanna, MPH, FAFPHM, Public Health Physician.
Department of Medicine, Mackay Base Hospital, Mackay, QLD.
Ian K Carney, FRACP, Staff Physician; currently Senior
Registrar, Department of Intensive Care, The Canberra Hospital,
ACT;
Joseph E Deverill, MB BS, MA, Registrar; currently
Department of Medicine, Nambour General Hospital, Nambour, QLD;
John A Botha, FRACP, Director; currently Director of
Intensive Care, Frankston Hospital, Frankston, VIC.
Public Health Virology, Queensland Health Scientific Services,
Coopers Plains, QLD.
Greg A Smith, PhD, Scientific Manager;
Ina L Serafin,
PhD, Research Coordinator;
Bruce J Harrower, BAppSc,
Scientist.
Department of Pathology, Mater Misericordiae Hospital, South
Brisbane, QLD.
Anthony E G Tannenberg, FRCPA, Neuropathologist.
Central Queensland Pathology Laboratories, Mackay, QLD.
Peter F Fitzpatrick, FRCPath, FRCPA, Histopathologist.
Division of Anatomical Pathology, Queensland Health Pathology
Service, Royal Brisbane Hospital, QLD.
Jeffrey W Searle, MD, FRCPA, Anatomical Pathologist.
Reprints: Dr J N Hanna, Tropical Public Health Unit,
Queensland Health, PO Box 1103, Cairns, QLD 4870.
md1AThealth.qld.gov.au
- Jeffrey N Hanna
- Ian K Carney
- Greg A Smith
- Joseph E Deverill
- John A Botha
- Ina L Serafin
- Bruce J Harrower
- Peter F Fitzpatrick
- Jeffrey W Searle
- 1.
- Hooper PT, Lunt RA, Gould AR, et al. A new lyssavirus -- the firstendemic rabies-related virus recognised in Australia. Bull InstPasteur 1997; 95: 209-218.
- 2.
- Gould AR, Hyatt AD, Lunt R, et al. Characterisation of a novellyssavirus isolated from Pteropid bats in Australia.Virus Res 1998; 54: 165-187.
- 3.
- Samaratunga H, Searle JW, Hudson N. Non-rabies Lyssavirus humanencephalitis from fruit bats: Australian bat Lyssavirus (pteropidLyssavirus) infection. Neuropathol Appl Neurobiol 1998;24: 331-335.
- 4.
- Helmick CG, Tauxe RV, Vernon AA. Is there a risk to contacts ofpatients with rabies? Rev Infect Dis 1987; 9: 511-518.
- 5.
- Lyssavirus Expert Group. Prevention of human lyssavirusinfection. Comm Dis Intell 1996; 20: 505-507.
- 6.
- Heaton PR, Johnstone P, McElhinney LM, et al. Heminested PCR assayfor detection of six genotypes of rabies and rabies-related viruses.J Clin Microbiol 1997; 35: 2762-2766.
- 7.
- Southern EM. Detection of specific sequences among DNA fragmentsseparated by gel electrophoresis. J Mol Biol 1975; 98:503-517.
- 8.
- Fishbein DB, Bernard KW. Rabies virus. In: Mandell GL, Bennett JE,Dolin R, editors. Principles and practice of infectious diseases.4th ed. New York: Churchill Livingstone, 1995: 1527-1543.
- 9.
- Jackson AC, Ye H, Phelan CC, et al. Extraneural organ involvement inhuman rabies. Lab Invest 1999; 79: 945-951.
- 10.
- Krebs JW, Smith JS, Rupprecht CE, Childs JE. Rabies surveillancein the United States during 1997. J Am Vet Med Assoc 1998; 213:1713-1728.
- 11.
- Barrett J, Young P, Field H, et al. Australian bat lyssavirus inQueensland. Abstracts of the Forty-eighth Annual Wildlife DiseaseAssociation Conference; 1999 Aug 8-12; Athens (Ga), abstract no 33.
- 12.
- Smith JS, Fishbein DB, Rupprecht CE, Clark K. Unexplained rabiesin three immigrants in the United States: a virologic investigation.N Engl Med J 1991; 324: 205-211.
- 13.
- Grattan-Smith PJ, O'Regan WJ, Ellis PSJ, et al. Rabies, a secondAustralian case, with a long incubation period. Med J Aust1992; 156: 651-654.
- 14.
- Wilde H, Choomkasien P, Hemachudha T, et al. Failure of rabiespostexposure treatment in Thailand. Vaccine 1989; 7: 49-52.
- 15.
- Rabies and Australian bat lyssavirus: recommendations for pre-and post-exposure vaccination. Comm Dis Intell 1998; 22:153.




