The clinical manifestations of osteoporosis affect nearly two million Australians.1 In the absence of interventions, the prevalence of osteoporosis-related conditions is predicted to increase over the next two decades from 10% of the population currently to 13.2% by 2021.1 The incidence of osteoporotic fractures is also predicted to increase, from one every 8.1 minutes in 2001 to one every 3.7 minutes in 2021.
Total costs relating to osteoporosis are currently estimated at $7.4 billion per annum, of which $1.9 billion are direct costs. Its disease burden can be expressed in terms of premature mortality and disability, which together represented over 25 000 healthy years of life lost to Australians in the financial year 2000–01.1
In Australia there are three ongoing prospective cohort studies of fracture epidemiology:
The Dubbo Osteoporosis Epidemiology Study (DOES) of a cohort of about 1600 men and 2100 women aged over 60 years with pre-fracture assessments;2
The Geelong Osteoporosis Study (GOS) of about 109 900 men and women aged over 35 years;3 and
The Tasmanian Older Adult Cohort (TASOAC) study of about 229 600 men and women of all ages.4
These studies provide different estimates of the number of fractures occurring in Australia. DOES reported 306 fractures in 3.25 years (1989–1992), giving an estimated residual lifetime fracture risk of 29% for men and 56% for women aged over 60.5 TASOAC reported 2140 fractures over two years (1997–1999), with an estimated residual lifetime fracture risk of 27% for men and 44% for women aged over 50.4 GOS reported 2184 fractures over two years (1994–1996), with an estimated lifetime risk of fracture of 42% in women aged over 503 (the estimate for men is not yet available). From these studies, the total number of fractures each year among Australians aged over 60 has been estimated at 73 000 (DOES), 57 000 (TASOAC) and 51 000 (GOS). Using a different methodology, Access Economics has estimated there were 65 000 osteoporotic fractures in Australia in 2001.1 Using the GOS estimates, it is calculated that the total number of hip fractures in Australia will increase from 15 000 in 1996 to 21 000 by 2006.6
The National Health and Medical Research Council (NHMRC) levels of evidence (see Box, this page) are a useful guide for defining the quality of available data on the treatment of osteoporosis. The most important criteria for a high-quality trial include randomisation, placebo controls, double-blinding, large sample sizes, prolonged observation, low dropout rates, preplanned intention-to-treat analyses, and replication. Replication and internal consistency are particularly important, as low event rates will often result in wide confidence intervals and type 2 errors (ie, failing to detect a difference when it is really present).
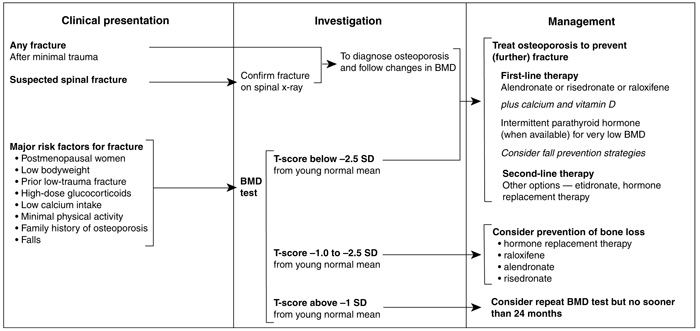
The presence of a spinal fracture is an indication that treatment should be given, provided that BMD is in the range for "osteoporosis" (T-score < –2.5) or "osteopenia" (T-score between –1 and –2.5) (see Box 1). If a non-spinal fracture is present, treatment should be considered if BMD is in the osteoporosis range (T-score < –2.5). However, prospective studies evaluating the antifracture efficacy of drugs in patients with osteoporosis and non-spinal fractures at baseline are not available. Women with osteoporosis (T-score < –2.5), with or without fractures, should be investigated and considered for treatment. Evidence in men is limited, and recommendations must await further research.
1: Explanation of T- and Z-scores, with World Health Organization thresholds 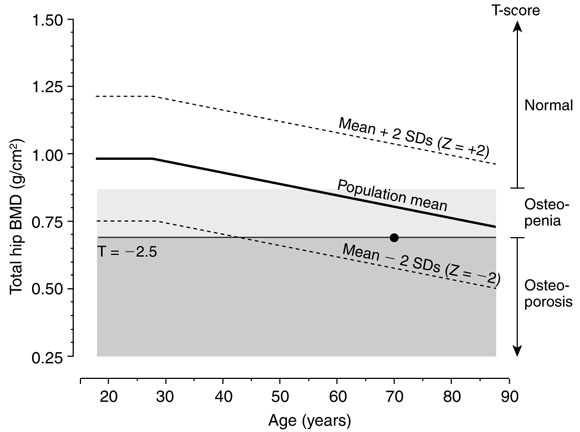
Relationship between hip bone mineral density (BMD) and age in women, showing the difference between: (i) Z-score (number of SDs from population mean for age). Z = –2.0 to +2.0 is the reference range; and (ii) T-score (number of SDs from mean for a young, healthy population). A T-score of –2.5 is defined as the threshold for osteoporosis.
• Indicates a woman aged 70 years with a BMD Z-score of –1, which is within the reference range for age. However, this Z-score means the woman has double the risk of fracture compared with a 70-year-old woman with mean BMD for age. Further, her T-score is –2.5, indicating her BMD is at the threshold for osteoporosis (adapted from Prince7).
Dual-energy x-ray absorptiometry (DEXA) is the current "gold standard" for the diagnosis of osteoporosis. BMD predicts fracture risk. Each standard deviation reduction in femoral-neck BMD increases the age-adjusted risk of hip fracture by a factor of about two (range, 2.0–3.5) and the risk of any atraumatic fracture by almost the same amount (range, 1.7–2.4).8 Similarly, each SD reduction in lumbar spine BMD increases the risk of spinal fracture by a factor of about 2.3 (range, 1.9–2.8). Proximal femur BMD appears to be the best overall predictor of fracture risk, particularly as it is unaffected by osteoarthritis, which can spuriously elevate spine BMD values.
BMD is expressed in terms of a "T-score", representing the number of SDs from the young normal mean BMD. Diagnosis based on bone densitometry, measured by DEXA and the T-score, provides a normal distribution of values as defined by a working group for the World Health Organization (see Box 1): 9
A BMD measurement should only be done if the decision to treat (or not to treat) is influenced by the result of the test. Thus, it is a valid and essential investigation in patients at high risk of osteoporotic fracture who seek medical advice, but not justified for screening a population of healthy people. At present, the decision to measure BMD in patients is supported by a Medicare rebate for certain high-risk categories only.10 The usefulness of population screening also depends on the prevalence of the disease and cost of the screening test. Screening of unselected populations (eg, using ultrasound in pharmacies) is not recommended by any authoritative group in the field of bone biology.
Moreover, the definition of osteoporosis is not without problems. The BMD cut-off of –2.5 SD below the young normal mean used to define osteoporosis was developed to apply to DEXA measurements of BMD at the spine or hip. This cut-off value, when applied to other techniques such as ultrasound, quantitative computed tomography or forearm measurements, does not identify the same number or the same proportion of individuals.11,12 Moreover, reference ranges may differ between people of different ethnic origin.
Several biochemical markers of bone turnover can be measured in serum and urine. Although measuring the levels of biochemical bone markers can not quantify total skeletal bone mass, it can provide additional information to assess fracture risk and may have a clinical role in measuring a patient's compliance and response to therapy. Serum and urine levels of several biochemical markers of bone resorption and formation have been used as surrogate endpoints for gauging drug efficacy. In women aged 75 years or older, urine C-telopeptide and free deoxypyridinoline crosslinks of type I collagen have been shown to be independent predictors of an increased risk of hip fracture, and their combination with low BMD is an even stronger predictor.13 Two other prospective studies, one of hip fractures in elderly women and the other of spinal and peripheral fractures in women closer to the menopause, have confirmed these observations.14,15 An elevated bone resorption marker in addition to low BMD strengthens the case for treatment in an individual. In clinical trials of HRT or bisphosphonates, the percentage decrease in bone turnover markers correlates with the change in BMD at two years.16,17 There is no good evidence that reduced levels of bone-turnover markers in response to therapy predict fracture-risk reduction.
Perhaps the single most easily recognised risk factor for osteoporotic fracture is the presence of any spinal18 or non-spinal fragility fracture. The risk for further spinal fractures increases 3–5-fold as the number or severity of prevalent deformities increases, rising to an 11-fold increase if three or more fractures are present. The risk of hip fracture increases after one or more spinal fractures. The risk of forearm fracture is higher if there has been a previous forearm fracture: of patients who had had a distal forearm fracture, 46% of women and 30% of men suffered further fractures over the following seven years.19
Trials of alendronate and raloxifene have been carried out in women who have osteoporosis but no prevalent fracture. Treatment reduces spinal fracture rates and, at least with bisphosphonates, the reduction in fracture rate is seen within 12–18 months.20,21
Data from alendronate studies indicate that a BMD T-score below –2.5 indicates a level of fracture risk comparable to that associated with a pre-existing low-trauma fracture. 21,22 The number needed to treat (NNT) to prevent a spinal fracture was 15 in women with a prior spinal fracture and 35 in women with low BMD without a prior fracture; for hip fracture, the NNTs were 81 and 90, respectively. Women with osteoporosis, even if they have no fractures, should be treated.
Should women with BMD T-scores between –1 and –2.5 but no prevalent fracture be treated? The issue in this group is that it comprises such a large proportion of women in the general population (estimated to be about 50% of all women over 50 years). In the Geelong Osteoporosis Study, 80% of the fractures occurred in women over 60 years; of the women under 60 years who had fractures, about 40% had osteoporosis and about 60% had normal BMD or BMD in the osteopenic range.3
Few data are available on the antifracture efficacy of drugs in women with osteopenia, as most trials have been carried out in women with osteoporosis. For this reason alone, making recommendations for treatment of women with osteopenia is difficult and not evidence-based, and making recommendations about universal treatment of large sectors of the population at modest risk for fracture is inappropriate. In RCTs, bisphosphonates have been shown to reduce by about 50% the risk of spinal fractures in women with BMD T-scores between –1 and –2.5, but this was not statistically significant, as event rates were low.20
Surveys of dietary calcium intake in Sydney, Dubbo and Geelong suggest that 75%–87% of premenopausal and postmenopausal women receive less than the recommended daily calcium intake of 800 mg/day (premenopausally) and 1000 mg/day (postmenopausally).23-25
Whether calcium intake or vitamin D status can be altered in the whole population, and whether this would reduce the population burden of fractures, is unknown. However, on the basis of current evidence,26 calcium and vitamin D supplementation is recommended for nursing-home residents in Australia.
In principle, drug therapy should be continued indefinitely, because stopping treatment results in increased remodelling, bone loss, progression of structural damage and increased fracture risk. Most of the increase in BMD that is observed occurs within the first two years of treatment, although continued increases have been reported with alendronate beyond that time. The longest study of bisphosphonates has been for seven years.27 If BMD increases into the normal range, it may be reasonable to consider stopping treatment and monitoring bone turnover markers and rates of bone loss. However, further research is needed to address the optimal duration of therapy.
Although there are deficiencies in our knowledge of the barriers to identification and treatment of osteoporosis in primary care, some areas that have been identified include a doctor's knowledge, perception and interpretation of diagnostic methods. In a study on diagnosis and treatment of osteoporosis by primary care physicians compared with specialists, the records of 1743 patients who had undergone bone densitometry scans were reviewed. The study revealed that primary care physicians were less likely to recognise and treat osteoporosis than specialist endocrinologists or rheumatologists, as they had had less exposure to specific education about osteoporosis.28
Despite evidence that the incidence of further fracture increases markedly after the first fracture, most people (over 80% in Australia) who present with their first osteoporotic fracture fail to be investigated or treated.29 Initiatives to increase rates of treatment, such as specialised multidisciplinary hospital-based first-fracture clinics, in cooperation with Divisions of General Practice, are one approach to this problem. Orthopaedic surgeons should heighten their awareness of the need for secondary prevention after a fracture in people aged over 50 years.
Barriers to identification, treatment and prevention of osteoporosis include inaccessibility of bone densitometry testing facilities and limited availability of subsidised medication. Patients and their treating practitioners require physical and financial access to bone densitometry testing facilities for effective clinical management. Current indications for bone densitometry under Medicare restrict access to densitometry,10 and PBS guidelines restrict the use of some agents to patients who have already had an osteoporotic fracture.
Densitometry testing has been subsidised by the MBS for selected indications since 1994. This followed a comprehensive evaluation by the National Health Technology Advisory Panel, with input from the medical profession, mainly the Australian and New Zealand Bone and Mineral Society and the Australian Medical Association. In the year 2000, Medicare funded 110 737 densitometry services in Australia at a cost of $7.6 million. There is a case for expanded indications for densitometry that might include patients at high risk of fracture, such as older patients, or people with a family history of spinal or hip fracture in a first-degree relative.30
For over 50 years, Australia has had in operation a subsidised scheme for pharmaceuticals as part of a comprehensive national health cover for all residents. Of all pharmaceutical expenditure in Australia (including prescription and over-the-counter products), the PBS system pays about 50%. The cost-effectiveness of medications is relevant to whether they are PBS-listed. The relative risk reduction in the incidence of new spinal fractures associated with most anti-osteoporotic medications is fairly consistent at around 50%, regardless of baseline risk or history of fracture.
As over 90% of hip fractures result from a fall, people found to be at high risk of falls are a group worthy of further investigation. Simple assessments for falls risk have been developed that discriminate (with sensitivities and specificities of 75%) between "faller" and "non-faller" groups living in the community and in institutions.31,32
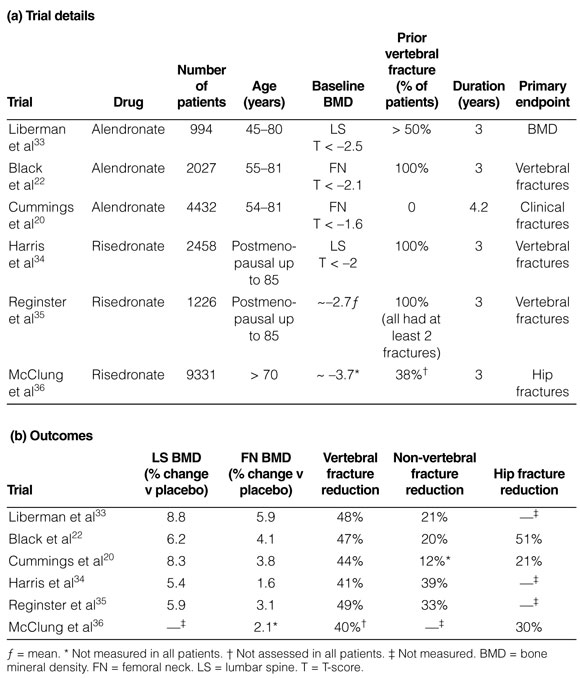
Three bisphosphonates are available in Australia for the treatment of osteoporosis: alendronate, risedronate and etidronate. Alendronate has been reported to reduce the risk of single and multiple spinal fractures, asymptomatic (morphometric) and symptomatic spinal fractures in women with osteoporosis and one or more baseline spinal fractures (E1).22,33 Risedronate has also been reported to reduce the risk of single and multiple spinal fractures and morphometric spinal fractures in women with osteoporosis and one or more baseline spinal fractures (E1) (see Box 2).34,35 Alendronate halves the risk of spinal fractures in women who have osteoporosis without a pre-existing spinal fracture.20 No studies with risedronate in this population are available. Although the BMD response and the suppression of bone remodelling with alendronate 70 mg once weekly is no different from alendronate 10 mg daily,38 there are no fracture studies with the latter formulation. Etidronate may also prevent spinal fractures (E2), but problems in design, execution, and analysis make the results of existing studies difficult to interpret.39,40
2: Major fracture-prevention randomised controlled trials with bisphosphonates in postmenopausal women with osteoporosis
The risk reduction with potent bisphosphonates is seen early, usually within 12 months. There is evidence that the reduction in spinal fracture risk with alendronate reduces bed-days, days of sickness and pain and healthcare costs.36
Non-spinal fracture rates are also reduced with alendronate and risedronate in patients with a prevalent spinal fracture (E1).33-35 Data for anti-hip-fracture efficacy are also available. In the alendronate trials there was consistency in hip-fracture risk reduction, but event rates were low and hip fracture was not a primary endpoint.20,22 In one risedronate trial,37 in which hip fractures were the primary endpoint and there were many events (232 hip fractures), there was a 40% reduction in hip-fracture risk among women aged 70–79 with a baseline femoral-neck T-score below –4 (or below –3 together with one non-skeletal risk factor for hip fracture) (E2). In women aged over 80 years and selected primarily on the basis of non-skeletal risk factors (such as poor gait or propensity to fall) but not low BMD, there was no significant reduction in hip-fracture risk overall. However, a recent analysis suggests that there was a reduction in intertrochanteric fractures in this group.41
Selective oestrogen-receptor modulators (eg, raloxifene) are compounds that have oestrogen agonist activity at some sites and antagonist activity at others. RCTs of the effects of raloxifene have shown increases in bone density,42 but less than those reported with bisphosphonates or oestrogen. Despite this, in postmenopausal women with osteoporosis, raloxifene treatment has been found to be associated with a 36% reduction in the risk of one or more spinal fractures using a 60 mg daily dose for four years.21 Non-spinal fractures were not reduced, for reasons that are unclear. Raloxifene treatment is also associated with a 60%–70% reduction in risk of breast cancer43 and with reduced low-density- lipoprotein cholesterol and total cholesterol (the latter being surrogate endpoints for cardioprotection).42
An increased risk of venous thrombosis has been reported with raloxifene, similar to that seen with oestrogen. Raloxifene should be stopped if patients are immobilised for any prolonged period. Unlike oestrogen, raloxifene is not useful for control of menopausal symptoms, and indeed may worsen them. Raloxifene has also been shown to be effective in preventing postmenopausal bone loss,44 and can be considered as an alternative in women unable to take oestrogen for this indication. Currently, there are no RCTs with preplanned endpoints supporting the use of selective oestrogen-receptor modulators for the prevention of non-spinal fractures.
Numerous RCTs have shown that oestrogen therapy can prevent bone loss in postmenopausal women (E1). Oestrogen is not only effective in preventing bone loss when given at or near menopause, but also continues to reduce bone loss for 10–15 years after menopause, with increases in bone density averaging 5% over three years.45,46
However, the paucity of trials demonstrating antifracture efficacy of HRT has led to questions about its value in the treatment of osteoporosis. Two recent meta-analyses of the effects of HRT on spinal and non-spinal fractures have reviewed 22 controlled trials of at least one year's duration in which HRT was compared with either placebo, no treatment, calcium, or vitamin D.47,48 In the pooled analysis, the relative risk of non-spinal fracture in women randomly assigned to receive HRT was 0.73 (95% CI, 0.56–0.94; P = 0.02). For hip and wrist fractures alone, the relative risk was 0.60 (95% CI, 0.40–0.91; P = 0.02). Significant effects were seen only in women under the age of 60.47 The finding that risk reduction was not significant in women over 60 was dependent on the inclusion of the HERS study,49 which involved relatively obese older women with cardiovascular disease who may not have had osteoporosis. For spinal fractures, the relative risk in women randomised to receive HRT was 0.67 (95% CI, 0.45–0.98; P = 0.04) and the effect was not confined to women under 60 years.48 Thus, although some positive data exist, there is a need for RCTs of the effect of HRT on spinal and non-spinal fractures.
Ideally, oestrogen therapy should be continuous, not cyclical, and long-term. Women with a uterus should take oestrogen in combination with progestogens to protect against endometrial cancer. Progestogens may be given cyclically for 10–14 days each month in perimenopausal women or as continuous therapy combined with oestrogen in postmenopausal women. The latter treatment is more suitable for women who are more than two years postmenopausal, to prevent the initial irregular bleeding (normally seen with this regimen) being unduly prolonged. Tibolone, a synthetic steroid reported to have activity through oestrogenic, progestogenic and androgenic receptors, can also improve bone density.50 Moreover, it can be taken without unwanted progestogen-induced withdrawal bleeding. However, there are no data evaluating its antifracture efficacy.
Controlled trials of calcium as a monotherapy have found small but consistent effects of calcium on BMD (E1), averaging 1%–2% over two to three years and showing accumulation over time.51,52 Several studies have reported a significant beneficial effect of calcium monotherapy on fracture incidence.51,53-55 However, these findings should be interpreted with caution, as the studies were small and not powered to assess the effects of calcium supplementation on fractures. They may represent selective reporting of fracture results in that fracture data were probably recorded in other RCTs but not reported because no significant effect was found. The effect of this bias towards the reporting of positive results will only be addressed when adequately powered studies with fracture rate as the primary endpoint are undertaken.
Vitamin D is better regarded as an endogenously produced pro-hormone than as an essential dietary constituent. It is produced in the skin as a result of sunlight exposure. With increasing age and frailty, vitamin D levels tend to decline, resulting in malabsorption of calcium and increased secretion of PTH, which in turn leads to accelerated bone loss. Physiological supplements of calciferol (eg, 400 IU/day) reduce PTH concentrations and lead to increases in BMD. Similar changes in biochemical endpoints can be achieved with regular sunlight exposure for 15–30 minutes daily. Two large studies have assessed the effect on fracture rates of calciferol supplementation alone. Lips et al56 reported no change in fracture incidence among 2578 community-dwelling men and women aged over 70 years who were randomised to receive calciferol 400 IU/day or placebo, whereas Heikinheimo et al57 reported that 150 000 IU/year of vitamin D reduced symptomatic fracture rates by 25% in a cohort of 800 elderly subjects in Finland.
Two other studies have reported the effects of calcium plus calciferol given to elderly people. Chapuy et al26 reported a reduction of more than 25% in non-spinal and hip fracture rates in a cohort of 3000 elderly institutionalised women studied over three years. Dawson-Hughes et al58 reported a reduction of more than 50% in non-spinal fracture rates among 400 older men and women randomised to receive calcium 500 mg/day plus 700 IU vitamin D per day, or placebo. It is not possible to determine whether the calcium, the vitamin D or the combination were the essential components in the success of these two studies. However, the studies point to the possibility that a safe and inexpensive intervention with calcium and vitamin D may reduce morbidity among institutionalised elderly patients.
A recent study of 489 postmenopausal women compared the effects of HRT and calcitriol over three years.59 HRT increased BMD by 3% at the femoral neck (P < 0.0001) and by 4.4% at the spine compared with placebo (P < 0.0001). By comparison, calcitriol had no significant effect on BMD at the femoral neck (0.1% increase; P = 0.57), but significantly increased BMD at the spine (1.7% increase; P = 0.01).
An open-label trial of 622 women found a threefold reduction in new spinal fractures among women with postmenopausal osteoporosis treated with calcitriol compared with women receiving supplemental calcium60 — fracture rates remained stable in calcitriol-treated patients but increased in the calcium-treated patients. This study suffered from several design flaws, making the results difficult to interpret (E3). In summary, the paucity of available data from well-designed, large-scale RCTs provides only weak evidence for the efficacy of calcitriol as a treatment for postmenopausal osteoporosis.
The largest study, involving 1637 postmenopausal women with prior spinal fractures, assigned participants randomly to receive placebo or one or two doses of subcutaneous recombinant human PTH over a median period of 19 months.61 New spinal fractures occurred in 14% of placebo-treated women versus 5% of women treated with 20 µg PTH. A 20 µg dose of PTH increased BMD by 9% (spine) and 3% (femoral neck) over and above the control group. Fracture-risk reduction with 20 µg PTH was 65% for spinal fractures and 55% for non-spinal fractures.
Androgenic steroids such as nandrolone have been widely used in Australia for management of osteoporosis. While there is some evidence of beneficial effects on bone density (E3),62 their antifracture efficacy is untested and there are no adequate safety data.
Recently, anabolic effects of statins on bone have been reported in vitro and in animal experiments. However, observational epidemiological studies of bone density and fracture rates among statin users are conflicting and may be confounded by the metabolic abnormalities that led to statin use in the first place. Two RCTs of statin use in non-osteoporotic populations have failed to demonstrate an effect of statins on fracture risk.63,64
There is a paucity of data on the effects of phytooestrogens on bone65,66 and no evidence that phyto-oestrogen supplements prevent bone loss. In one prospective, randomised, placebo-controlled study of 474 women treated with ipriflavone, no significant differences in BMD, biochemical bone markers or spinal fracture rates were observed after 36 months.67
Risk factors for falls include impairments of vision, sensation, strength and balance, and patient thinness and frailty. In the Dubbo Osteoporosis Epidemiology Study, quadriceps strength and postural sway were of similar importance to BMD in predicting fractures in both men and women.2 Most fractures occur after falls, but not all falls result in fractures. Nevertheless, interventions that reduce falls risk may prevent fractures.
Several studies have examined single and multiple risk factors and the use of hip protectors. Of the single risk factor interventions, programs that involve balance training, such as home-based physiotherapy and tai chi, reduce the risk of falls (pooled relative risk [RR], 0.80; 95% CI, 0.66–0.98; and RR, 0.51; 95% CI, 0.36–0.75, respectively).68,69 Environmental modifications by occupational therapists (eg, removing mats, improving lighting) may reduce falls.70 One study has reported that a reduction in psychoactive medications reduces the risk of falls (RR, 0.34; 95% CI, 0.16– 0.74), but adoption and compliance rates in the study were low.71
Studies in nursing homes in Denmark and Sweden of the effect of hip protectors have reported reductions in hip fracture rates of 56% and 67%, respectively (E2).72,73 However, hip fractures did occur in the intervention subjects when not wearing their hip protectors, so that compliance remains an issue (one reason for non-compliance is that women believe it makes them look "fat" around the hips). Systematic reviews74 suggest that hip protectors reduce the risk of hip fracture in high-risk populations (E1).
Vigorous exercise undertaken by athletes during growth is well documented as increasing peak bone mass by biologically worthwhile amounts.75-77 The difference in bone density between athletes and sedentary controls ranges from 5% to 25%, depending on the sport and duration of participation. After retirement from intensive training, the effects appear to persist for many years, but whether the benefits are maintained into old age, when fractures and falls become common, is uncertain. The sparse data on 70–80- year-old retired athletes suggest that the effects may be eroded in people who have substantially reduced training volumes. The same pattern is seen for other physiological adaptations to exercise, such as muscle hypertrophy, increased aerobic capacity, and increased insulin sensitivity.
Whether moderate exercise during growth produces benefits in terms of BMD and bone structure is less well established. There have been several exercise studies in school children in which loading (such as jumping and other sporting activity) is incorporated into the physical education program for 20–30 minutes three times weekly. The results are generally positive, with variable evidence of increased bone size, increased BMD and thickening of cortices.78-80 However, there are few data on whether the modest improvements in bone mass and structure are maintained after these exercise programs are stopped. There have been no long-term (> two years) exercise intervention studies using moderate exercise programs in normally active children.
Moderate- to high-intensity weight-bearing aerobic exercise, high-intensity progressive resistance training and high-impact loading (such as jumping) increase BMD by 1%–4% in pre- and postmenopausal women (E1).81-85 Excessive exercise carries some risk, especially in premenopausal women, in whom it may induce amenorrhoea. In studies of generally one year's duration, with sample sizes ranging from 30 to 150, exercise has been found to slow the rate of bone loss in older women by about 1.5% per year compared with sedentary controls (E1).86 More robust exercise interventions appear to produce greater effects, but optimal prescriptive elements await further RCTs. Inclusion of weight-lifting and balance-training exercises should provide the widest range of benefits relevant to fracture protection, as well as reducing muscle weakness, falls risk and depression, and increasing muscle mass and mobility. Whether these benefits translate into fracture-risk reduction is currently unknown.
There is little evidence available regarding pain management after osteoporotic spinal fracture. The general principles of management of acute, subacute and chronic pain include use of non-pharmacological modalities and recognition of the potential for comorbid mood disorders, particularly in elderly people. Non-pharmacological, non-evidence-based modalities include physiotherapy and other physical modalities, transcutaneous electrical nerve stimulation, cognitive behaviour therapy, and procedures such as vertebroplasty, kyphoplasty and nerve blockade. Pharmacotherapy should employ a stepwise approach in the use of analgesics and other pain-modifying agents. Subcutaneous calcitonin has been reported to reduce the pain of acute spinal fractures in two small placebo-controlled trials (E2).87,88 Rehabilitation to independent living is the primary goal after any fracture.
Rehabilitation after hip fracture has been the most investigated. Systematic reviews of randomised and non-randomised trials89,90 have concluded that coordinated geriatric hip-fracture programs and early discharge (with support) for selected patients can significantly increase rates of returning home and reduce length of hospital stay and costs (E1). No controlled trials are available to guide recommendations specifically for spinal fractures. Given the absence of evidence for this condition, strategies that encourage independence and limitation of disability, together with interventions directed at secondary prevention of fractures, should be applied in clinical practice.
While there are multiple published RCTs assessing the benefits of different therapies for osteoporosis in postmenopausal women, studies of osteoporosis in other populations (such as men, glucocorticoid-treated patients, and frail older people) are relatively few. Here we attempt to summarise the evidence for treatments in these "neglected" populations, using the same NHMRC levels of evidence. Evidence and recommendations regarding children with osteoporosis and athletes with stress fractures can be found at the Osteoporosis Australia website (<http://www.osteoporosis.org.au>).
Osteoporotic fractures occur in about 28% of men aged over 60 years.5 While fractures tend to occur in elderly men with multiple comorbid disorders, secondary underlying causes of osteoporosis are common and need to be rigorously excluded. Up to 16% of men with spinal fractures have evidence of hypogonadism. Chronic smoking, excessive alcohol use, glucocorticoid therapy, malabsorption and underlying bone-marrow malignancies are some of the important risk factors for osteoporosis that need to be identified.
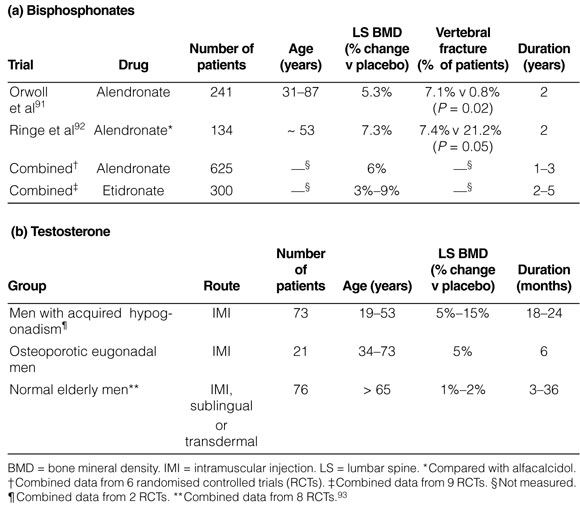
Osteoporosis remains a neglected area in men's health, with less than 10% of men with osteoporotic fractures currently receiving antifracture therapy. RCTs of men with osteoporosis are generally of smaller sample size and lesser quality than in those involving postmenopausal women (see Box 3). The limited RCTs that have been conducted suggest that alendronate, followed by cyclical etidronate, are the drugs of choice for men with primary osteoporosis. 91,92,94 The effects of risedronate have not yet been reported, except in men receiving corticosteroids, but its efficacy should be similar to that of alendronate and etidronate. Preliminary studies also suggest that subcutaneous PTH may be as effective in reducing fracture rates in men as it is in postmenopausal women.
Adequate supplementation with calcium and vitamin D (if required) is recommended for all men with osteoporosis. There are no RCTs assessing the role of calcium or vitamin D3 alone in men. In a single small RCT (20 treated subjects and 19 controls) in osteoporotic men with pre-existing fractures conducted over two years,95 calcitriol was no better than calcium in reducing spinal fracture (E3).
Testosterone replacement therapy is indicated for men with hypogonadism (serum total testosterone concentration < 8 nmol/L), but there are no data to assess the antifracture efficacy of testosterone in men with osteoporosis. Testosterone therapy and its effect on BMD are largely dependent on the gonadal and growth status of the individual, the duration of pre-existing hypogonadism, the degree of osteopenia and the duration of testosterone therapy. Two RCTs (E2) have demonstrated the positive effects of testosterone therapy on both cortical and trabecular bone, with maximal responses occurring in men before epiphyseal closure.96,97 The results of studies of testosterone therapy in hypogonadal, osteoporotic eugonadal and normal elderly men are summarised in Box 3.96-100
A number of RCTs of bisphosphonates, in which the primary efficacy endpoint was BMD, have shown a consistent reduction in spinal fracture risk in postmenopausal women taking glucocorticoids (E1).101-105 In these studies, the risk of spinal fracture in control-group women taking glucocorticoids ranged from 13% to 22% over 12 months. For etidronate, alendronate and residronate, the number of postmenopausal women taking glucocorticoids who would need to be treated to prevent one fracture over 12 months was low. Treating these women would be more cost-effective than treating postmenopausal women with osteoporosis unrelated to glucocorticoid use.
A number of RCTs of active vitamin-D metabolites, such as calcitriol and alfacalcidol, have reported prevention of spinal bone loss in patients starting glucocorticoids.106,107 None of these studies were powered for fracture as an endpoint, and mild hypercalcaemia occurred in about 10%–20% of patients.
In trials in which calcium alone was used as the control therapy for patients starting glucocorticoids, calcium did not prevent rapid spinal bone loss.106,108 However, in patients receiving chronic low-dose glucocorticoids, treatment with calcium and simple vitamin D resulted in small increases in spine BMD.109 Although none of these studies were powered for fracture as an endpoint, meta-analyses have concluded that adjunctive therapy with some form of vitamin D should be considered.110
There is limited evidence for the effects of HRT (either oestrogen or testosterone) on bone density (E2), and there are no data on the effectiveness of HRT in reducing fractures among people with glucocorticoid-induced osteoporosis. Nevertheless, HRT should probably be considered if hypogonadism is present.111,112
Older people are more likely to have several risk factors for fracture, including previous fractures. NNT analyses suggest that older people are more likely to derive greater benefit per year of anti-osteoporotic treatment than younger people.113 However, in frail older subjects, osteoporosis treatments must take account of the likelihood of comorbidity and the use of multiple other therapies. Calcium and vitamin D supplementation may have a special role in treating older frail people, particularly those in residential care. In Australia, 22% of women in low-level care and 45% of women in high-level care have frank vitamin D deficiency, and virtually all the remainder have a 25-hydroxy-vitamin-D level in the lower half of the reference range.114 Other factors that need special consideration in frail older people are the use of hip protectors and interventions to prevent falls.
The most rigorously investigated drugs in the field of osteoporosis are the potent bisphosphonates alendronate and risedronate, and the selective oestrogen-receptor modulator raloxifene (see Box 4). Calcium, in combination with vitamin D, has been reported to reduce fracture risk in nursing-home residents and ambulant individuals.
Abbreviations
DEXA dual energy x-ray absorptiometry
DOES Dubbo Osteoporosis Epidemiology Study
GOS Geelong Osteoporosis Study
HRT hormone replacement therapy
MBS Medicare Benefits Schedule
NHMRC National Health and Medical Research Council
PBS Pharmaceutical Benefits Scheme
Philip N Sambrook, MD, LLB, FRACP
Professor of Rheumatology, Institute of Bone and Joint Research, University of Sydney
Stephen R Phillips, MB BS, FAMA
Peter R Ebeling, MD, FRACP
Shona L Bass, PhD, MSc
Senior Lecturer, School of Health Sciences, Deakin University, Victoria
Kim L Bennell, BAppSc, PhD
Ian D Cameron, MB BS, PhD
Chris T Cowell, MB BS, FRACP
Clinical Associate Professor, Institute of Endocrinology, The Children's Hospital at Westmead
Susan R Davis, MB BS, FRACP, PhD
Terry Diamond, MB BCh, FRACP
John A Eisman, AO, PhD, FRACP
Leon Flicker, MB BS, PhD
Professor of Geriatric Medicine, Royal Perth Hospital, University of Western Australia
Linda R Ferris, MB BS, BSc(Med), FRACS(Orth)
Maria A Fiatarone Singh, MD, FRACP
Professor of Medicine, John Sutton Chair of Exercise and Sport Science, University of Sydney
Paul P Glasziou, MB BS, PhD
Michael J Hooper, MB BS, FRACP
Clinical Associate Professor, Department of Medicine, Concord Hospital, Sydney
Graeme Jones, MD, FRACP
Associate Professor, and Head, Musculoskeletal Unit, Menzies Research Institute, Hobart
Stephen R Lord, PhD
Lyn M March, PhD, FRACP
Sheila M O'Neill, MB BCh, BAO, MICGP
Clinical Director of Research, Betty Byrne Henderson Centre, Royal Women's Hospital, Brisbane
Nick A Pocock, MD, FRACP
Richard L Prince, MD, FRACP
Ian R Reid, MD, FRACP
Professor of Medicine and Endocrinology, Department of Medicine, University of Auckland, New Zealand
Kerrie M Sanders, MHN, PhD
John D Wark, PhD, FRACP
Professor of Medicine, Department of Medicine, University of Melbourne, and Royal Melbourne Hospital
- 1. The burden of brittle bones: costing osteoporosis in Australia. Canberra: Access Economics, 2001.
- 2. Nguyen T, Sambrook PN, Kelly P, et al. Prediction of osteoporotic fractures by postural instability and bone density. BMJ 1993; 307: 1111-1115.
- 3. Sanders KM, Seeman E, Ugoni AM, et al. Age and gender specific rate of fractures in Australia: a population based study. Osteoporos Int 1999; 10: 240- 247.
- 4. Cooley H, Jones G. A population based study of fracture incidence in southern Tasmania: lifetime fracture risk and evidence for geographic variations within the same country. Osteoporos Int 2001; 12: 124-130.
- 5. Jones G, Nguyen T, Sambrook PN, et al. Symptomatic fracture incidence in elderly men and women: the Dubbo Osteoporosis Epidemiology Study. Osteoporos Int 1994; 4: 277-282.
- 6. Sanders KM, Nicholson GC, Ugoni AM, et al. Health burden of hip and other fractures in Australia beyond 2000. Projections based on the Geelong Osteoporosis Study. Med J Aust 1999; 170: 467-470. <eMJA full text>
- 7. Prince RL. Diagnosing osteoporosis: the value of quantitative ultrasound. Med J Aust 1999; 171: 295-296. <eMJA full text>
- 8. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteporotic fractures. BMJ 1996; 312: 1254-1259.
- 9. Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 1994; 4: 368-381.
- 10. Medicare Benefits Schedule Book. Canberra: Commonwealth Department of Aged Care, 2001.
- 11. Faulkner KG, von Stetten E, Miller P. Discordance in patient classification using Tscores. J Clin Densitom 1999; 2: 343-350.
- 12. Henry MJ, Pasco JA, Nicholson GC, et al. Prevalence of osteoporosis in Australian women: Geelong Osteoporosis Study. J Clin Densitom 2000; 3: 261- 268.
- 13. Garnero P, Hausherr E, Chapuy M-C, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS prospective study. J Bone Miner Res 1996; 11: 1531-1538.
- 14. van Daele PLA, Seibel MK, Burger H, et al. Case-control analysis of bone resorption markers, disability, and hip fracture risk: the Rotterdam study. BMJ 1996; 312: 482-483.
- 15. Riis BJ, Overgaard K, Christiansen C. Biochemical markers of bone turnover to monitor the bone mass response to postmenopausal hormone replacement therapy. Osteoporos Int 1995; 5: 276-280.
- 16. Hannon R, Blumsohn A, Naylor K, Eastell R. Response of biochemical markers of bone turnover to hormone replacement therapy: impact of biological variability. J Bone Miner Res 1998;13: 1124-1133.
- 17. Greenspan SL, Parker RA, Ferguson L, et al. Early changes in biochemical markers of bone turnover predict the long-term response to alendronate therapy in representative elderly women: a randomized clinical trial. J Bone Miner Res 1998; 13: 1431-1438.
- 18. Ross PD, Davis JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture in women. Ann Intern Med 1991; 114: 919-923.
- 19. Cuddihy MT, Gabriel SE, Crowson CS, et al. Forearm fractures as predictors of subsequent osteoporotic fractures. Osteoporos Int 1999; 9: 469-475.
- 20. Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures. JAMA 1998; 280: 2077-2082.
- 21. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3 year randomised controlled trial. JAMA 1999; 282: 637-645.
- 22. Black DM, Cummings SR, Karpf D, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996; 348: 1535-1541.
- 23. Angus RM, Sambrook PN, Pocock NA, Eisman JA. Dietary intake and bone mineral density. Bone Miner 1988; 4: 265-277.
- 24. Pasco JA, Sanders KM, Henry MJ, et al. Calcium intakes among Australian women: Geelong Osteoporosis Study. Aust N Z J Med 2000; 30: 21-27.
- 25. Nguyen TV, Center JR, Eisman JA. Osteoporosis in elderly men and women: effects of dietary calcium, physical activity, and body mass index. J Bone Miner Res 2000; 15: 322-331.
- 26. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 1993; 327: 1637-1642.
- 27. Tonino RP, Meunier PJ, Emkey R, et al. Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. J Clin Endocrinol Metab 2000; 85: 3109-3115.
- 28. Saadi H, Litaker D, Mills W, et al. Practice variation in the diagnosis and treatment of osteoporosis: a case for more effective physician education in primary care. J Womens Health Gend Based Med 1999; 8: 767-771.
- 29. Zochling JM, Schwarz JM, March L, Sambrook PN. Is osteoporosis undertreated after minimal trauma fracture? [letter] Med J Aust 2001; 174: 663-664.
- 30. Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. N Engl J Med 1995; 332: 767-773.
- 31. Lord SR, Clark RD, Webster IW. Physiological factors associated with falls in an elderly population. J Am Geriatr Soc 1991; 39: 1194-1200.
- 32. Lord SR, Ward JA, Williams P, Anstey K. Physiological factors associated with falls in older community-dwelling women. J Am Geriatr Soc 1994; 42: 1110-1117.
- 33. Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med 1995; 333: 1437-1443.
- 34. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and non-vertebral fractures in women with postmenopausal osteoporosis. JAMA 1999; 282: 1433-1452.
- 35. Reginster JY, Minne HW, Sorensosen OH, et al, Randomised trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int 2000; 11: 83-91.
- 36. McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med 2001; 344: 333-340.
- 37. Schnitzer T, Bone HG, Crepaldi S, et al. Therapeutic equivalence of alendronate 70 mg once weekly and alendronate 10 mg daily in the treatment of osteoporosis. Aging 2000; 12: 1-12.
- 38. Harris ST, Watts NB, Jackson RD, et al. Four-year study of intermittent cyclic etidronate treatment of postmenopausal osteoporosis — three years of blinded therapy followed by one year of open therapy. Am J Med 1993; 95: 557-567.
- 39. Storm T, Thamsborg G, Steiniche T, et al. Effect of intermittent cyclical etidronate therapy on bone mass and fracture rate in postmenopausal osteoporosis. N Engl J Med 1990; 322: 1265-1271.
- 40. Nevitt MC, Thompson DE, Black DM, et al. Effect of alendronate on limitedactivity days and bed-disability days caused by back pain in postmenopausal women with existing vertebral fractures. Arch Intern Med 2000; 160: 77-85.
- 41. Eastell R, McClung M, Reginster JY, et al. Efficacy of risedronate in decreasing the incidence of femoral neck and intertrochanteric fractures in older women with osteoporosis [abstract]. J Bone Miner Res 2001; 16(Suppl 1): S219.
- 42. Delmas PD, Bjarnason BH, Mitlak NH, et al. Effect of raloxifene on bone mineral density, serum cholesterol and uterine endometrium in postmenopausal women. N Engl J Med 1997; 337: 1641-1647.
- 43. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4 year results from the MORE trial. Breast Cancer Res Treat 2001; 65: 125-134.
- 44. Johnell O, Scheele WH, Lu Y, et al. Additive effects of raloxifene and alendronate on bone density and biochemical measurements of bone remodeling in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 2002. In press.
- 45. Ettinger B, Genant HK, Cann CE. Long-term estrogen replacement therapy prevents bone loss and fractures. Ann Intern Med 1985; 102: 319-324.
- 46. Writing group for the PEPI trial. Effects of hormone therapy on bone mineral density. JAMA 1996; 276: 1389-1396.
- 47. Torgerson DJ, Bell-Syer SE. Hormone replacement therapy and prevention of non-vertebral fractures: a meta-analysis of randomized trials. JAMA 2001; 285: 2891-2897.
- 48. Torgerson DJ, Bell-Syer SE. Hormone replacement therapy and prevention of vertebral fractures: a meta-analysis of randomised trials. BMC Musculoskelet Disord 2001; 2: 7-10.
- 49. Cauley JA, Black DM, Barrett-Connor E, et al. Effects of hormone replacement therapy on clinical fractures and height loss: the Heart and Estrogen/Progestin Replacement Study (HERS). Am J Med 2001; 110: 442-450.
- 50. Studd J, Amala I, Kicovic PM, et al. A randomised study of tibolone on bone mineral density in osteoporotic postmenopausal women with previous fractures. Obstet Gynecol 1998; 92: 574-579.
- 51. Reid IR, Ames RW, Evans MC, et al. Effect of calcium supplementation on bone loss in postmenopausal women. N Engl J Med 1993; 328: 460-464.
- 52. Nordin BEC. Calcium and osteoporosis. Nutrition 1997; 13: 664-686.
- 53. Chevalley T, Rizzoli R, Nydegger V, et al. Effects of calcium supplements on femoral bone mineral density and vertebral fracture rate in vitamin D replete elderly patients. Osteoporos Int 1994; 4: 245-252.
- 54. Recker RR, Hinders S, Davies KM, et al. Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res 1996; 11: 1961- 1966.
- 55. Cumming RG, Nevitt MC. Calcium for prevention of osteoporotic fractures in postmenopausal women. J Bone Miner Res 1997; 12: 1321-1329.
- 56. Lips P, Graafmans WC, Ooms ME, et al. Vitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo-controlled clinical trial. Ann Intern Med 1996; 124: 400-406.
- 57. Heikinheimo RJ, Inkovaara JA, Harju EJ, et al. Annual injection of vitamin D and fractures of aged bones. Calcif Tissue Int 1992; 51: 105-110.
- 58. Dawson-Hughes B, Harris S, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997; 337: 670-676.
- 59. Gallagher JC, Fowler SE, Detter JR, Sherman SS. Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J Clin Endocrinol Metab 2001; 86: 3618-3628.
- 60. Tilyard MW, Spears GF, Thomson J, Dovey S. Treatment of postmenopausal osteoporosis with calcitriol or calcium. N Engl J Med 1992; 326: 357-362.
- 61. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344: 1434-1441.
- 62. Flicker L, Hopper JL, Larkins RG, et al. Nandrolone decanoate and intranasal calcitonin as therapy in established osteoporosis. Osteoporos Int 1997; 7: 29-35.
- 63. Reid IR, Hague W, Emberson J, et al. Effect of pravastatin on frequency of fracture in the LIPID study: secondary analysis of a randomised controlled trial. Lancet 2001; 357: 509-512.
- 64. Chan KA, Andrade SE, Boles M, et al. Inhibitors of hydroxymethylglutarylcoenzyme A reductase and risk of fracture among older women. Lancet 2000; 355: 2185-2188.
- 65. Alekel DL, Germain AS, Peterson CT, et al. Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am J Clin Nutr 2000; 72: 844-852.
- 66. Potter SM, Baum JA, Teng H, et al. Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am J Clin Nutr 1998; 68(6 Suppl): 1375S-1379S.
- 67. Alexandersen P, Toussaint A, Christiansen C, et al. Ipriflavone in the treatment of postmenopausal osteoporosis: a randomized controlled trial. JAMA 2001; 285: 1482-1488.
- 68. Gillespie LD, Gillespie WJ, Robertson MC, et al. Interventions for preventing falls in elderly people (Cochrane Review). The Cochrane Library. Issue 4. Oxford: Update Software, 2001.
- 69. Wolf SL, Barnhart HX, Kutner NG, et al. Reducing frailty and falls in older persons: an investigation of Tai Chi and computerized balance training. J Am Geriatr Soc 1996; 44: 489-497.
- 70. Cumming R, Thomas M, Szonyi G, et al. Home visits by an occupational therapist for assessment and modification of environmental hazards: a randomized controlled trial of falls prevention. J Am Geriatr Soc 1999; 47: 1397-1402.
- 71. Campbell AJ, Robertson MC, Gardner MM, et al. Psychotropic medication withdrawal and a home based exercise program to prevent falls: results of a randomised controlled trial. J Am Geriatr Soc 1999; 47: 850-853.
- 72. Ekman A, Mallmin H, Michaelsson K, Ljunghall S. External hip protectors to prevent osteoporotic hip fractures. Lancet 1997; 350: 563-564.
- 73. Lauritzen JB, Petersen MM, Lund B. Effect of external hip protectors on hip fractures. Lancet 1993; 341: 11-13.
- 74. Parker MJ, Gillespie LD, Gillespie WJ. Hip protectors for preventing hip fractures in the elderly (Cochrane Review). The Cochrane Library. Issue 4. Oxford: Update Software, 2001.
- 75. Karlsson M, Bass S, Seeman E. The evidence that exercise during growth or adulthood reduces the risk of fragility fractures is weak. Best Pract Res Clin Rheumatol 2001; 15: 429-450.
- 76. Bass S, Pearce G, Bradney M, et al. Exercise before puberty may confer residual benefits in bone density in adulthood: studies in active prepubertal and retired female gymnasts. J Bone Miner Res 1998; 13: 500-507.
- 77. Huddleston A, Rockwell D, Kulund DN, Harrison B. Bone mass in lifetime tennis athletes. JAMA 1980; 244: 1107-1109.
- 78. Fuchs RK, Bauer JJ, Snow CM. Jumping improves hip and lumbar spine bone mass in prepubescent children: a randomized controlled trial. J Bone Miner Res 2001; 16: 148-156.
- 79. McKay H, Petit MA, Schutz RW, et al. Augmented trochanteric bone mineral density after modified physical education classes: a randomised school-based exercise intervention study in prepubescent and early pubescent children. J Pediatr 2000; 136(2): 156-162.
- 80. Bass S, Delmas PD, Pearce G, et al. The differing tempo of growth in bone size, mass and density in girls is region-specific. J Clin Invest 1999; 104: 795-804.
- 81. Snow-Harter C, Bouxsein ML, Lewis BT, et al. Effects of resistance and endurance exercise on bone mineral status of young women: a randomized exercise intervention trial. J Bone Miner Res 1992; 7: 761-769.
- 82. Lohman T, Going S, Pamenter R, et al. Effects of resistance training on regional and total bone mineral density in premenopausal women: a randomized prospective study. J Bone Miner Res 1995; 10: 1015-1024.
- 83. Wolff I, Croonenborg J, Kemper H, et al. The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int 1999; 9: 1-12.
- 84. Prince RL, Smith M, Dick IM, et al. Prevention of postmenopausal osteoporosis. A comparative study of exercise, calcium supplementation, and hormone-replacement therapy. N Engl J Med 1991; 325: 1189-1195.
- 85. Kelley G, Kelley D, Kristi S, Tran Z. Resistance training and bone mineral density in women: a meta-analysis of controlled trials. Am J Phys Med Rehabil 2001; 80: 65-77.
- 86. Wallace BA. Cumming RG. Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcif Tissue Int 2000; 67: 10-18.
- 87. Lyritis GP, Tsakalakos N, Magiasis B, et al. Analgesic effect of salmon calcitonin in osteoporotic vertebral fractures: a double blind placebo controlled clinical study. Calcif Tissue Int 1991; 49: 369-372.
- 88. Pun KK, Chan MB. Analgesic effect of intranasal salmon calcitonin in the treatment of osteoporotic vertebral fractures. Clin Ther 1989; 11: 205-209.
- 89. Cameron ID, Crotty M, Currie C, et al. Geriatric rehabilitation following fractures in older people: a systematic review. Health Technol Assess 2000; 4(2): 1-111.
- 90. Ruchlin HS, Elkin EB, Allegrante JP. The economic impact of a multifactorial intervention to improve postoperative rehabilitation of hip fracture patients. Arthritis Rheum 2001; 45: 446-452.
- 91. Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med 2000; 343: 604-610.
- 92. Ringe JD, Faber H, Dorst A. Alendronate treatment of established primary osteoporosis in men: results of a 2-year prospective study. J Clin Endocrinol Metab 2001; 86: 5252-5255.
- 93. Diamond T, Sambrook P, Williamson M, et al. Guidelines for treatment of osteoporosis in men. Aust Fam Physician 2001; 30: 787-791.
- 94. Anderson FH, Francis RM, Bishop J, Rawlings DJ. Effect of intermittent cyclical disodium etidronate therapy on bone mineral density in men with vertebral fractures. Age Ageing 1997; 26: 359-365.
- 95. Ebeling PR, Wark JD, Yeung S, et al. Effects of calcitriol or calcium on bone mineral density, bone turnover, and fractures in men with primary osteoporosis: a two-year randomized, double blind, double-placebo study. J Clin Endocrinol Metab 2001; 86: 4098-4103.
- 96. Devogelaer JP, De Cooman S, Nagant de Deuxchaisnes C. Low bone mass in hypogonadal males: effect of testosterone substitution therapy, a densitometric study. Maturitas 1992; 15: 17-23.
- 97. Katznelson L, Finkelstein JS, Schoenfeld DA, et al. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab 1996; 81: 4358-4365.
- 98. Francis RM. The effects of testosterone on osteoporosis in men. Clin Endocrinol 199; 50: 411-414.
- 99. Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 1999; 84: 2647-2653.
- 100. Leifke E, Korner HC, Link TM, et al. Effects of testosterone replacement therapy on cortical and trabecular bone mineral density, vertebral body area and paraspinal muscle strength in hypogonadal men. Eur J Endocrinol 1998; 138: 51-58.
- 101. Adachi JD, Bensen WG, Brown J, et al. Intermittent etidronate therapy to prevent corticosteroid-induced osteoporosis. N Engl J Med 1997; 337: 382-387.
- 102. Saag K, Emkey R, Schnitzler TJ, et al. Alendronate for the prevention and treatment of glucocorticoid induced osteoporosis. N Engl J Med 1998; 339: 292- 299.
- 103. Cohen S, Levy RM, Keller M, et al. Residronate therapy prevents corticosteroidinduced bone loss. Arthritis Rheum 1999; 42: 2309-2318.
- 104. Reid DM, Hughes RA, Laan RF, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomised trial. J Bone Miner Res 2000; 15: 1006-1013.
- 105. Homik JE, Cranney A, Shea B, et al. A meta-analysis on the use of bisphosphonates in corticosteroid induced osteoporosis. J Rheumatol 1999; 26: 1148-1157.
- 106. Sambrook PN, Birmingham J, Kelly PJ, et al. Prevention of corticosteroid osteoporosis: a comparison of calcium, calcitriol and calcitonin. N Engl J Med 1993; 328: 1747-1752.
- 107. Reginster JY, Kuntz D, Verdicht W, et al. Prophylactic use of alfacalcidol in corticosteroid-induced osteoporosis. Osteoporos Int 1999; 9: 75-81.
- 108. Adachi J, Bensen W, Bianchi F, et al. Vitamin D and calcium in the prevention of corticosteroid-induced osteoporosis: a three year follow up study. J Rheumatol 1996; 23: 995-1000.
- 109. Buckley LM, Leib ES, Cartularo KS, et al. Calcium and vitamin D3 supplementation prevents bone loss in the spine secondary to low dose corticosteroids in patients with rheumatoid arthritis. Ann Intern Med 1996; 125: 961-968.
- 110. Amin S, LaValley MP, Simms RW, Felson DT. The role of vitamin D in corticosteroid induced osteoporosis: a meta-analytic approach. Arthritis Rheum 1999; 42: 1740-1751.
- 111. Hall GM, Daniels M, Doyle DV, Spector TD. The effect of hormone replacement therapy on bone mass in rheumatoid arthritis treated with and without steroids. Arthritis Rheum 1994; 37: 1499-1505.
- 112. Reid IR, Wattie DJ, Evans MC, Stapleton JP. Testosterone therapy in glucocorticoid-treated men. Arch Intern Med 1996; 156: 1173-1177.
- 113. Ensrud KE, Black DM, Palermo L, et al. Treatment with alendronate prevents fractures in women at highest risk: results from the fracture intervention trial. Arch Intern Med 1997; 157: 2617-2624.
- 114. Flicker L, Mead K, Nowson C, et al. Risk factors for falls in older women in residential care in Australia. J Bone Miner Res 2001; 16(Supp1): S170.





All members of the Writing Group and the Working Group signed a conflict of interest declaration. These are available from Professor Sambrook.