Being transgender or gender diverse (TGD) is now viewed as part of the natural spectrum of human diversity.1 Estimates suggest that 0.1–2% of the population are TGD.2 TGD people are individuals whose gender identity is markedly and persistently incongruent with their sex assigned at birth. They often experience intense body dysphoria driving individuals to seek gender‐affirming hormone therapy to align physical characteristics with gender identity. Stigma and discrimination contribute to poor mental health. Australian data demonstrate that over 50% have medically diagnosed depression and are at high risk of suicide.3,4,5
The World Health Organization International Classification of Diseases 11th Revision has recently declassified gender incongruence as a mental health disorder, with a goal to decrease stigma and social exclusion.6 Nonetheless, as those with gender incongruence have specific health needs, an understanding of diagnostic criteria can be useful (Box 1). A detailed discussion of gender terms and gender identity has been outlined in the Australian standards of care for TGD children and adolescents.7
While many TGD individuals will identify with a binary gender (ie, transgender male or transgender female), about 30% identify with a non‐binary gender (Box 2).3
Rapid increases in demand for transgender health services have recently been reported worldwide.3,8 Although international clinical practice guidelines exist,9,10 recommendations are based on low level evidence, broad and open to interpretation. Additionally, there are differences internationally with availability, subsidy and access to medications. Medical training in transgender health care is lacking and 79% of Australian clinicians experienced in prescribing gender‐affirming hormone therapy supported the development of local guidelines.11 As such, we aim to provide specific recommendations for the hormonal and related management of TGD individuals aged over 18 years for Australian medical practitioners.
Methods
During the 2017 Australian Professional Association for Trans Health (AusPATH, formerly ANZPATH) Biennial Conference, the need for an Australian‐based gender‐affirming hormone‐treatment pathway was highlighted. A working group was formed, chaired by the first author (AC). Members identified relevant evidence, published guidelines and expert opinion to develop the overview. There is an absence of randomised controlled trials in the field. Recommendations are based on low or very low level evidence and expert opinion, with authors placing a high value on harm minimisation and clinical need. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework has been used for key recommendations.12 This approach classifies recommendations as strong (1) or weak (2) and evidence quality as high (A), moderate (B), low (C) or very low (D).12 A survey of Australian TGD adult individuals was conducted to ascertain health needs, and results informed these guidelines. Australian prescribing patterns among the AusPATH membership were also ascertained by survey.11 Controversies were resolved by discussion within the group. The draft statement was submitted to TGD community members, the AusPATH executive, the Endocrine Society of Australia (ESA) Medical Affairs Committee and the Royal Australasian College of Physicians (RACP) Policy and Advocacy Committee for feedback. The ESA and RACP invited external expert reviewers to provide comments, which were incorporated. The final version was endorsed by AusPATH, the ESA and the RACP.
Recommendations
Caring for gender diverse patients: general
Establishing and affirming an individual's gender identity and using the name and pronoun the person uses are vital for consultations and correspondence; legal identity markers can be used for Medicare purposes (GRADE: 1D). In addition to “she” or “he”, gender diverse individuals may use other pronouns (eg, “they”) or no pronoun. Consideration should be given to gender neutral bathrooms and gender inclusive registration forms.
Management of TGD individuals ideally involves a multidisciplinary team (general practitioner, nurse specialist, psychologist, psychiatrist, endocrinologist, sexual health physician, gynaecologist, surgeon and speech pathologist as appropriate). Trained peer support workers can improve mental distress through facilitating access to support services, and provide advice on non‐medical aspects of gender transition.
Not all who identify as TGD desire medical intervention. Many undergo social transition and change gender expression without medical intervention. Hormonal therapy with estradiol or testosterone will feminise or masculinise physical characteristics to align with an individual's gender identity (Box 3). Individual goals, especially for non‐binary people, may be complex and treatment should be individualised; however, tailoring hormonal therapy to attain some characteristics and not others can be challenging.
Mental health review
A patient‐centred yet holistic informed consent model of care is valid and Australian protocols exist.13 These suggest a comprehensive mental health review by an experienced clinician (GP, physician, psychiatrist or psychologist) with adequate time to assess and support individuals seeking transition, to ensure that mental health concerns are appropriately treated and transition‐related mood changes or stressors are managed10,11 (GRADE: 1D). Mental health professionals play an important role in more complex cases, and can affirm capacity for informed consent and exclude less common conditions such as psychosis, dissociative identity disorder or body dysmorphic disorder.
Medical assessment
TGD individuals commonly self‐report avoidance of medical care due to fear of discrimination.14 A request for hormonal therapy can be an opportunity to provide routine medical care and preventive screening in addition to gender‐affirming care. Relative contraindications to testosterone or estradiol therapy (such as polycythaemia, thrombosis, liver disease or cardiac failure) should be considered. There are insufficient data regarding the long term effects of hormonal therapy on cardiovascular outcomes.15 A retrospective audit suggested that the most common cause of increased mortality in TGD people was cardiovascular disease.16 Weight gain17 and lipid derangements15 may occur in individuals commencing hormone therapy, and smoking increases the risk of venous thrombosis at commencement of hormone therapy.18 As a harm minimisation approach, we suggest assessing and mitigating cardiovascular risk factors.
Thrombosis risk may influence choice of estradiol preparation. Retrospective studies report a 5% incidence of venous thrombosis during estradiol therapy in TGD women, and incidence was highest with ethinyl estradiol use.19,20 Thrombosis risk is greatest in the first year of treatment, and with smoking, obesity and increasing age (> 40 years).21 Post‐menopausal data suggest that transdermal estrogens carry minimal thrombotic risk.22 Those at high risk should consider transdermal preparations and if thrombosis occurs, they should consider concurrent anticoagulation.22
In patients desiring hormone therapy, we recommend the following baseline investigations:11
- full blood examination — testosterone raises haematocrit levels and lowering testosterone lowers haematocrit levels;23
- liver function tests — estradiol is poorly metabolised in the setting of hepatic impairment; no changes in liver enzymes have been observed in transgender males;24
- electrolytes — hyperkalemia, although uncommon with normal renal function, can occur with spironolactone;
- fasting lipids and glucose — testosterone therapy lowers high density lipoprotein cholesterol levels, and raises triglyceride and low density lipoprotein cholesterol levels;15 the effect of testosterone on insulin resistance is unclear,25,26 although estradiol may worsen insulin resistance;25
- estradiol and total testosterone (see below).
Information regarding sexually transmitted infections, including human immunodeficiency virus pre‐exposure prophylaxis, should be provided based on individual risk indicators.27
Chromosomal analyses are rarely abnormal in TGD individuals28 and should only be performed if there is clinical suspicion (eg, Klinefelter syndrome). Genital examination is not routinely required.
While sex steroids are important in bone metabolism, a recent meta‐analysis of TGD individuals showed no adverse effect on bone density.29 Sex steroid deficiency due to pubertal suppression or following gonadectomy may accelerate bone loss. International guidelines recommend that bone mineral density measurement be considered in individuals with risk factors for osteoporosis, including subtherapeutic hormonal replacement.10
Medical therapy
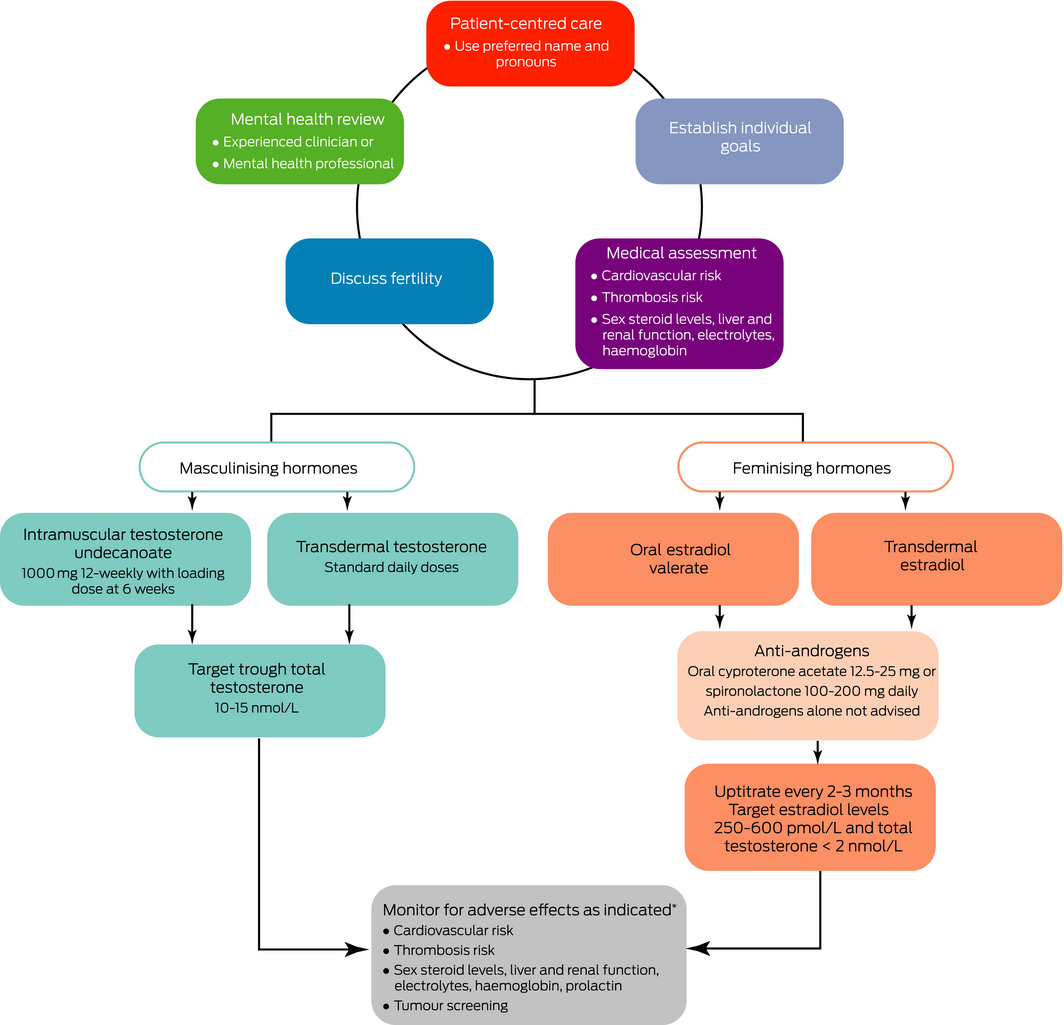
Gender‐affirming hormonal therapy in cohort and cross‐sectional studies appears to improve psychological functioning, quality of life, depression and suicidal ideation30,31 (GRADE: 1C). A suggested algorithm is shown in Box 3.
Before hormonal treatment, we suggest that patients should understand the expected physical changes and time course of effects (Box 4), potential adverse effects (Box 5), and the irreversible nature of some changes (eg, voice lowering with testosterone). Most effects begin within a few months but maximal effects may take 2–3 years.
Hormonal therapy can impair fertility and patients should receive counselling. Sperm cryopreservation should be discussed before estradiol therapy due to expected changes in spermatogenesis. Oocyte storage can be considered; however, ovulation typically resumes on cessation of testosterone therapy. Testosterone is a teratogen and does not always prevent ovulation, so contraception should be discussed.
Masculinising hormone therapy. Standard replacement doses of testosterone are recommended to initiate masculinisation (GRADE: 1C). Doses can be adjusted to target trough total testosterone levels in the lower end of the male reference interval (10–15 nmol/L) (GRADE: 2D). The Pharmaceutical Benefits Scheme (PBS) criteria for androgen deficiency apply if gender markers are male or female. For people requiring masculinising hormone therapy for gender dysphoria, we use the authority indication “androgen deficiency due to an established testicular disorder”. The patient must be treated by or in consultation (including teleconsult, phone or email) with a paediatrician, endocrinologist, urologist or sexual health physician. The specialist's name must be given in the authority application. Gender markers can be male or female.
The following testosterone formulations are available under the PBS:
- testosterone undecanoate 1000 mg, intramuscularly administered 12‐weekly (with the first two doses 6 weeks apart);
- testosterone 1% (50 mg/5 g) gel sachets, applied transdermally, one sachet daily;
- testosterone 1% (12.5 mg/actuation) gel in pump pack, applied transdermally, four actuations daily — this preparation can be easily titrated;
- testosterone 5% (50 mg/mL) cream 2 mL, applied transdermally daily.
Testosterone enantate and testosterone esters are also non‐PBS subsidised options.
Masculinising procedures. Testosterone therapy is highly effective at masculinising external appearance. Individuals may also desire surgery.4 Options include:
- bilateral chest reconstruction mastectomy (colloquially known as “top surgery”);
- hysterectomy ± oophorectomy;
- metoidioplasty (clitoral release and urethral lengthening — “bottom surgery”);
- phalloplasty (penis creation — “bottom surgery”).
Complication rates from metoidioplasty and phalloplasty are significant and outcomes may be suboptimal.38 Gender‐affirming surgery in the public sector in Australia is limited.
Chest binding is a common practice to tightly compress chest tissue, hiding the appearance of breasts. Severe skin irritation, pain, bruising and fractured ribs can result. Correctly sized, commercially purchased binders are recommended. Binders should be removed for sleep and use limited to 8–12 hours per day.39
Feminising hormone therapy. Estradiol therapy can be administered transdermally or orally. No data exist on gradual versus rapid titration or comparison of formulations in TGD individuals. Replicating female puberty, where estradiol levels gradually rise over 2 years, commencing at low doses with gradual uptitration every 2–3 months, is reasonable.10 Typical full doses are oral estradiol or estradiol valerate 2–6 mg daily and transdermal estradiol patches 100–150 μg/24 hours changed twice weekly (GRADE: 1C). The adhesion of transdermal patches can be challenging in warm climates and in individuals with body hair.
Treatment should be adjusted based on clinical response; however, feminisation is typically slow (GRADE: 2D). The value of biochemical monitoring is uncertain; when performed, trough estradiol levels should be used. International guidelines recommend target estradiol levels 367–734 pmol/L; however, this is not based on any supportive data.10 An Australian audit of 81 TGD individuals who had received estradiol therapy for over 6 months found that the mean estradiol level was 290 pmol/L with a median oral estradiol valerate dose of 6 mg daily.40 We recommend targeting estradiol levels of 250–600 pmol/L and total testosterone levels < 2 nmol/L (ie, in the pre‐menopausal female reference range) (GRADE: 2D). Individuals who wish to maintain erectile function may desire higher levels of testosterone; however, this will offset feminising effects.
High dose ethinyl estradiol (100 μg daily) was used until 1989 when retrospective audits showed increased risk of thromboembolic disease and possibly elevated risk of cardiovascular death.41 These data underpin suggestions to avoid ethinyl estradiol.
Progestins. Despite anecdotal reports that progestins increase breast growth, no data support their use. Healthy post‐menopausal women who received estradiol with progestins had increased risk of coronary heart disease compared with placebo34 (not reported with estradiol alone42). Progestins can also increase risk of thrombosis, bloating, nausea and weight gain and are not recommended.10 Cyproterone acetate, a commonly used anti‐androgen agent, has progestogenic effects.
Estradiol injections and implants. Therapeutic Goods Administration‐approved estradiol injections and implants are not available in Australia. Estradiol injections or implants obtained from compounding pharmacies currently lack testing for potency, efficacy, safety and quality control.43
Anti‐androgen therapy. Anti‐androgens are often required in addition to estradiol therapy to lower endogenous testosterone levels or inhibit testosterone effects. Spironolactone (100–200 mg daily) or cyproterone acetate (12.5–25 mg daily) are both effective.11 There are no comparative data. Both inhibit peripheral testosterone effects, but cyproterone acetate is also a potent progestin, suppressing gonadotrophins and testosterone production.44 Cyproterone acetate may lower mood; however, it is unclear whether this is related to the drug, suppressed testosterone levels or interaction with the glucocorticoid receptor.45 Case reports of meningioma46 and prolactinoma35 (or transient rises in serum prolactin32) have occurred with high dose cyproterone acetate (100–200 mg), and the lowest effective dose should be used.
Gonadotrophin‐releasing hormone analogues are used as puberty blockers in adolescents, subsidised by specialist paediatric gender services. Due to lack of PBS subsidy, costs can be prohibitive.
Feminising procedures. Surgical options for feminisation include:
- vaginoplasty and orchidectomy (“bottom surgery”);
- bilateral orchidectomy alone;
- breast augmentation (“top surgery”) — breast development with estradiol can take up to 3 years but remains suboptimal in many;47
- facial feminisation surgery;
- chondrolaryngoplasty reduces the thyroid cartilage;
- laryngoplasty and vocal cord surgery can aid voice feminisation;
- estradiol therapy is typically ceased peri‐operatively to avoid risk of thromboembolism; anti‐androgens are not required following orchidectomy.
Voice training. Voice and communication are important aspects of gender expression and can contribute to dysphoria, particularly if vocal pitch results in misgendering.48 Speech pathologists can provide feminising or masculinising voice training.
Facial and body hair. Changes to hair growth patterns from hormonal therapy can be slow due to hair follicle lifespan.49 Permanent hair removal (laser or electrolysis) is often required. Preferred methods depend on hair colour and site, with advice best provided by hair removal professionals.
Genital tucking. Tucking is a frequent practice to minimise the appearance of the penis and scrotum. Similar to chest binding, skin irritation, infection, pain and bruising can result. Specialty designed garments or medical tape may alleviate risks; however, no data exist on the safety of tucking for prolonged periods.
Monitoring and support
While rigorous long term studies are required, retrospective cohort studies suggest that short term gender‐affirming hormone therapy is safe, and significant benefits on mental health outweigh potential risks (GRADE: 1C). In the first year of treatment, 3‐monthly monitoring is suggested to review clinical effects, sex steroid levels, mood changes and adverse effects, and provide general preventive screening10,11 (GRADE: 2C). Mental health and spiritual and peer support can be beneficial during transition. Once stable, individuals can be reviewed less frequently (6–12 monthly). Weight gain may occur when commencing hormone therapy17 and lifestyle advice is recommended. Smoking cessation should be encouraged. Cancer screening should be individualised based on the presence of organs in TGD individuals, not gender identity or hormonal therapy status.35
Polycythaemia with testosterone therapy
Haemoglobin levels should be compared with the male reference interval. If the haematocrit level is > 0.5,10 exclude alternative causes (eg, smoking) and consider decreasing the testosterone dose or increasing the dosing interval.
Persistent menstruation on testosterone therapy
Menstrual suppression usually occurs within 1–6 months of testosterone therapy, but menses can continue beyond 12 months.50 If menses result in significant dysphoria, options include increasing testosterone levels, oral progestins or progestin‐releasing intrauterine devices.
Acne with testosterone therapy
Acne peaks at 6 months and gradually improves over time.51 Topical retinoids or retinoid–benzoyl peroxide combinations are useful for mild to moderate acne. Moderate to severe acne may require oral antibiotics or isotretinoin.
Summary
Increasing numbers of TGD individuals are seeking health care in Australia and clinicians need to provide appropriate gender‐affirming care. While pathways to gender transition are individualised, hormonal therapy is effective at aligning physical characteristics with gender identity and improving dysphoria, quality of life and mental health. Further medical research is needed to guide clinical care and understand the long term effects of hormonal therapies.
Box 1 – ICD‐11 diagnostic criteria for gender incongruence of adolescence or adulthood
Gender incongruence of adolescence and adulthood is characterised by a marked and persistent incongruence between an individual's experienced gender and the assigned sex, as manifested by at least two of the following:- a strong dislike of or discomfort with one's primary or secondary sex characteristics (in adolescents, anticipated secondary sex characteristics) due to their incongruity with the experienced gender
- a strong desire to be rid of some or all of one's primary and/or secondary sex characteristics (in adolescents, anticipated secondary sex characteristics) due to their incongruity with the experienced gender
- a strong desire to have the primary and/or secondary sex characteristics of the experienced gender.
Box 2 – Distinction between gender identity, gender expression and sex assigned at birth
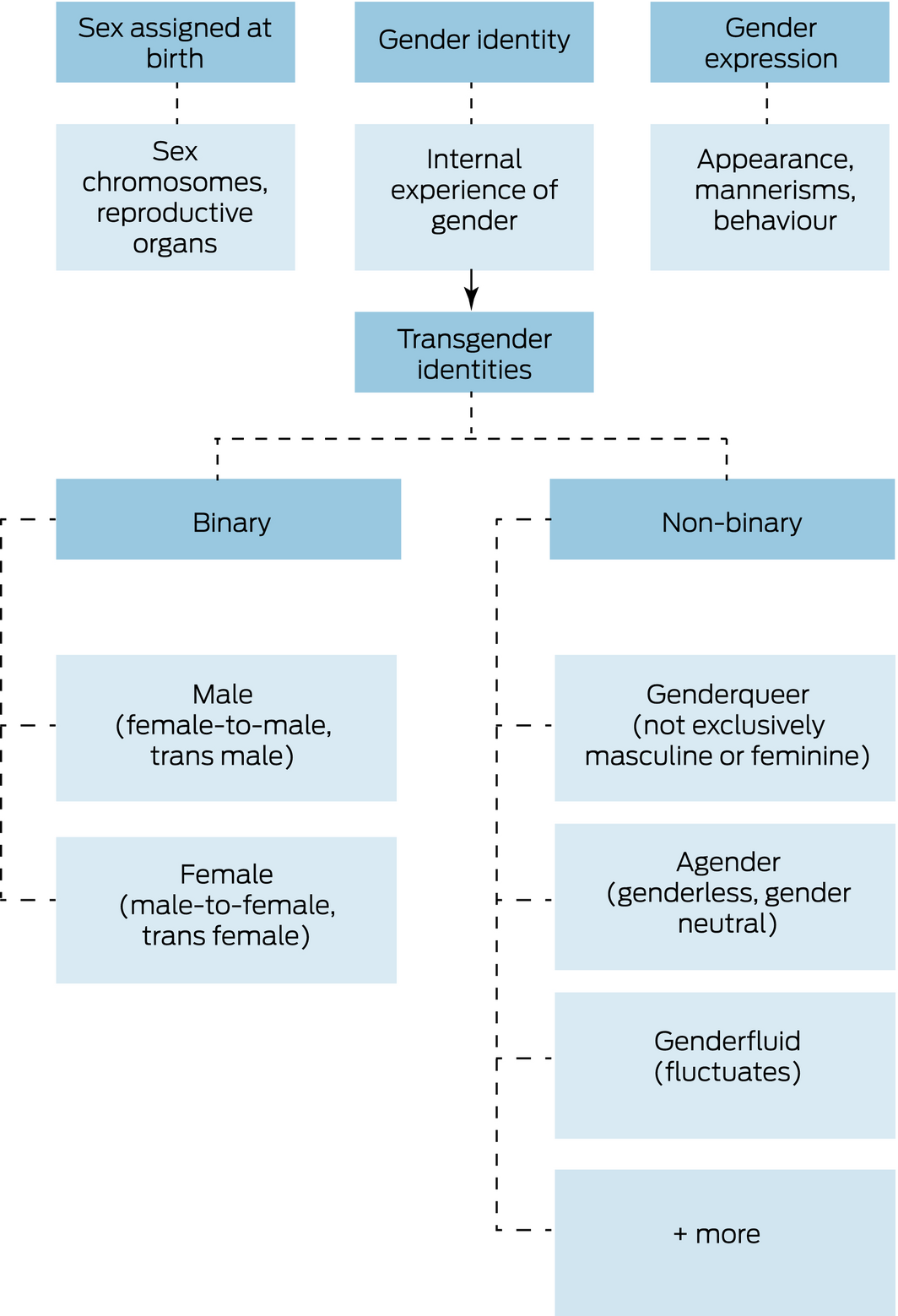
Gender identity and gender expression are distinct from biological sex. Although there are many gender identities,7 transgender and gender diverse identities can be roughly separated into binary (ie, trangender male, transgender female) and non‐binary. The term non‐binary is used here as a broad umbrella category to describe identities which are outside of the binary; however, non‐binary is also a specific gender identity.
Box 4 – Effects of feminising and masculinising hormone therapy
|
Feminising hormone effects |
Masculinising hormone effects |
||||||||||||||
|
|
|||||||||||||||
|
Body fat redistribution |
Body fat redistribution |
||||||||||||||
|
Decreased muscle mass |
Increased muscle mass |
||||||||||||||
|
Softening of skin/decreased oiliness |
Oily skin and acne |
||||||||||||||
|
Decreased libido |
Increased libido |
||||||||||||||
|
Decreased spontaneous erections |
Facial/body hair growth |
||||||||||||||
|
Male sexual dysfunction |
Male pattern baldness |
||||||||||||||
|
Breast growth |
Deepened voice |
||||||||||||||
|
Decreased testicular volume |
Vaginal atrophy |
||||||||||||||
|
Decreased sperm production |
Clitoral enlargement |
||||||||||||||
|
Decreased growth of body and facial hair |
Cessation of menses |
||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 5 – Possible risks and side effects of gender‐affirming hormone treatment*
|
Type of therapy |
Potential risks |
||||||||||||||
|
|
|||||||||||||||
|
Estradiol |
Thromboembolic disease19 |
||||||||||||||
|
Hypertriglyceridaemia15 |
|||||||||||||||
|
Prolactin elevation32 |
|||||||||||||||
|
Gall bladder disease33 |
|||||||||||||||
|
Breast cancer† |
|||||||||||||||
|
Testosterone |
Polycythaemia23 |
||||||||||||||
|
Acne36 |
|||||||||||||||
|
Sleep apnoea37 |
|||||||||||||||
|
Dyslipidaemia (increased triglyceride and LDL levels; decreased HDL levels)15 |
|||||||||||||||
|
|
|||||||||||||||
|
HDL = high density lipoprotein; LDL = low density lipoprotein. * This table provides an overview of risks associated with estradiol and testosterone therapy, some of which are extrapolated from cisgender populations. Long term data in TGD individuals are lacking. † An increased risk of breast cancer is seen with post‐menopausal estrogen therapy in cisgender women, which increases with duration of use.34 Owing to a lack of data, there are uncertainties regarding the risks of other hormone‐dependent tumours35 and cardiovascular disease.15 |
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
- Ada S Cheung1
- Katie Wynne2
- Jaco Erasmus3
- Sally Murray4
- Jeffrey D Zajac1
- 1 Austin Health, University of Melbourne, Melbourne, VIC
- 2 Diabetes and Endocrinology, Hunter New England Health, Newcastle, NSW
- 3 Gender Clinic, Monash Health, Melbourne, VIC
- 4 Sexual Health Service, Royal Perth Hospital, Perth, WA
The figure in Box 2 was designed for this article by graphic artist Jake Kidson‐Purry. We thank TGD community members who reviewed and provided feedback on the position statement: Daria Chetcuti, Max Rainier, Alex Wong andTransgender Victoria committee members. We also thank expert reviewers Ruth McNair (University of Melbourne), Michael Irwig (George Washington University, USA), the AusPATH executive committee, the ESA Medical Affairs Committee and the RACP Policy and Advocacy Committee for reviewing the manuscript. Ada Cheung is supported by an Australian Government National Health and Medical Research Council Early Career Fellowship (#1143333) and receives research support from the Viertel Charitable Foundation Clinical Investigator Award, an ESA Postdoctoral Award and the RACP Vincent Fairfax Family Foundation.
No relevant disclosures.
- 1. Saraswat A, Weinand JD, Safer JD. Evidence supporting the biologic nature of gender identity. Endocr Pract 2015; 21: 199–204.
- 2. Goodman M, Adams N, Cornell T, et al. Size and distribution of transgender and gender nonconforming populations: a narrative review. Endocrinol Metab Clin North Am 2019; 48: 303–321.
- 3. Cheung AS, Ooi O, Leemaqz S, et al. Sociodemographic and clinical characteristics of transgender adults in Australia. Transgend Health 2018; 3: 229–238.
- 4. Hyde Z, Doherty M, Tilley PJM, et al. The First Australian National Trans Mental Health Study: summary of results. Perth: School of Public Health, Curtin University, 2014. https://www.beyondblue.org.au/docs/default-source/research-project-files/bw0288_the-first-australian-national-trans-mental-health-study-summary-of-results.pdf (viewed Mar 2019).
- 5. Strauss P, Cook A, Winter S, et al. Trans Pathways: the mental health experiences and care pathways of trans young people. Summary of results. Perth: Telethon Kids Institute, 2017. https://www.telethonkids.org.au/globalassets/media/documents/brain-behaviour/trans-pathwayreport-web2.pdf (viewed Mar 2019).
- 6. World Health Organization. ICD‐11: Classifying disease to map the way we live and die. WHO: Geneva, 2018. https://www.who.int/health-topics/international-classification-of-diseases (viewed June 2018).
- 7. Telfer MM, Tollit MA, Pace CC, Pang KC. Australian standards of care and treatment guidelines for transgender and gender diverse children and adolescents. Med J Aust 2018; 209: 132–136. https://www.mja.com.au/journal/2018/209/3/australian-standards-care-and-treatment-guidelines-transgender-and-gender
- 8. Delahunt JW, Denison HJ, Sim DA, et al. Increasing rates of people identifying as transgender presenting to Endocrine Services in the Wellington region. N Z Med J 2018; 131: 33–42.
- 9. Coleman E, Bockting W, Botzer M, et al. Standards of care for the health of transsexual, transgender, and gender‐nonconforming people, version 7. Int J Transgenderism 2012; 13: 165–232.
- 10. Hembree WC, Cohen‐Kettenis PT, Gooren L, et al. Endocrine treatment of gender‐dysphoric/gender‐incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2017; 102: 3869–3903.
- 11. Bretherton I, Thrower E, Grossmann M, et al. Cross‐sex hormone therapy in Australia: the prescription patterns of clinicians experienced in adult transgender healthcare. Intern Med J 2019; 49: 182–188.
- 12. Alhazzani W, Guyatt G. An overview of the GRADE approach and a peek at the future. Med J Aust 2018; 209: 291–292. https://www.mja.com.au/journal/2018/209/7/overview-grade-approach-and-peek-future
- 13. Equinox Gender Diverse Health Centre. Protocols for the initiation of hormone therapy for trans and gender diverse patients. Melbourne: Thorne Harbour Health (formerly Victorian AIDS Council), 2017. http://www.transmedicalresearch.org/health-professionals (viewed Jan 2019).
- 14. Samuels EA, Tape C, Garber N, et al. “Sometimes you feel like the freak show”: a qualitative assessment of emergency care experiences among transgender and gender‐nonconforming patients. Ann Emerg Med 2018; 71: 170–182.e1.
- 15. Maraka S, Singh Ospina N, Rodriguez‐Gutierrez R, et al. Sex steroids and cardiovascular outcomes in transgender individuals: a systematic review and meta‐analysis. J Clin Endocrinol Metab 2017; 102: 3914–3923.
- 16. Simonsen RK, Hald GM, Kristensen E, Giraldi A. Long‐term follow‐up of individuals undergoing sex‐reassignment surgery: somatic morbidity and cause of death. Sex Med 2016; 4: e60–e68.
- 17. Fisher AD, Castellini G, Ristori J, et al. Cross‐sex hormone treatment and psychobiological changes in transsexual persons: two‐year follow‐up data. J Clin Endocrinol Metab 2016; 101: 4260–4269.
- 18. Ott J, Kaufmann U, Bentz EK, et al. Incidence of thrombophilia and venous thrombosis in transsexuals under cross‐sex hormone therapy. Fertil Steril 2010; 93: 1267–1272.
- 19. Wierckx K, Elaut E, Declercq E, et al. Prevalence of cardiovascular disease and cancer during cross‐sex hormone therapy in a large cohort of trans persons: a case‐control study. Eur J Endocrinol 2013; 169: 471–478.
- 20. van Kesteren PJ, Asscheman H, Megens JA, Gooren LJ. Mortality and morbidity in transsexual subjects treated with cross‐sex hormones. Clin Endocrinol (Oxf) 1997; 47: 337–342.
- 21. Canonico M, Plu‐Bureau G, Lowe GD, Scarabin PY. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta‐analysis. BMJ 2008; 336: 1227–1231.
- 22. Shatzel JJ, Connelly KJ, DeLoughery TG. Thrombotic issues in transgender medicine: a review. Am J Hematol 2017; 92: 204–208.
- 23. Defreyne J, Vantomme B, Van Caenegem E, et al. Prospective evaluation of hematocrit in gender‐affirming hormone treatment: results from European Network for the Investigation of Gender Incongruence. Andrology 2018; 6: 446–454.
- 24. Velho I, Fighera TM, Ziegelmann PK, Spritzer PM. Effects of testosterone therapy on BMI, blood pressure, and laboratory profile of transgender men: a systematic review. Andrology 2017; 5: 881–888.
- 25. Elbers JM, Giltay EJ, Teerlink T, et al. Effects of sex steroids on components of the insulin resistance syndrome in transsexual subjects. Clin Endocrinol (Oxf) 2003; 58: 562–571.
- 26. Cupisti S, Giltay EJ, Gooren LJ, et al. The impact of testosterone administration to female‐to‐male transsexuals on insulin resistance and lipid parameters compared with women with polycystic ovary syndrome. Fertil Steril 2010; 94: 2647–2653.
- 27. Wright E, Grulich A, Roy K, et al. Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine HIV pre‐exposure prophylaxis: clinical guidelines. Update April 2018. J Virus Erad 2018; 4: 143–159.
- 28. Inoubli A, De Cuypere G, Rubens R, et al. Karyotyping, is it worthwhile in transsexualism? J Sex Med 2011; 8: 475–478.
- 29. Singh‐Ospina N, Maraka S, Rodriguez‐Gutierrez R, et al. Effect of sex steroids on the bone health of transgender individuals: a systematic review and meta‐analysis. J Clin Endocrinol Metab 2017; 102: 3904–3913.
- 30. White Hughto JM, Reisner SL. A systematic review of the effects of hormone therapy on psychological functioning and quality of life in transgender individuals. Transgend Health 2016; 1: 21–31.
- 31. Tucker RP, Testa RJ, Simpson TL, et al. Hormone therapy, gender affirmation surgery, and their association with recent suicidal ideation and depression symptoms in transgender veterans. Psychol Med 2018; 48: 2329–2336.
- 32. Defreyne J, Nota N, Pereira C, et al. Transient elevated serum prolactin in trans women is caused by cyproterone acetate treatment. LGBT Health 2017; 4: 328–336.
- 33. Cirillo DJ, Wallace RB, Rodabough RJ, et al. Effect of estrogen therapy on gallbladder disease. JAMA 2005; 293: 330–339.
- 34. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 2002; 288: 321–333.
- 35. McFarlane T, Zajac JD, Cheung AS. Gender‐affirming hormone therapy and the risk of sex hormone‐dependent tumours in transgender individuals: a systematic review. Clin Endocrinol (Oxf) 2018; 89: 700–711.
- 36. Motosko CC, Zakhem GA, Pomeranz MK, Hazen A. Acne: a side‐effect of masculinizing hormonal therapy in transgender patients. Br J Dermatol 2019; 180: 26–30.
- 37. Cole AP, Hanske J, Jiang W, et al. Impact of testosterone replacement therapy on thromboembolism, heart disease and obstructive sleep apnoea in men. BJU Int 2018; 121: 811–818.
- 38. Nikolavsky D, Hughes M, Zhao LC. Urologic complications after phalloplasty or metoidioplasty. Clin Plast Surg 2018; 45: 425–435.
- 39. Peitzmeier S, Gardner I, Weinand J, et al. Health impact of chest binding among transgender adults: a community‐engaged, cross‐sectional study. Cult Health Sex 2017; 19: 64–75.
- 40. Cheung AS, Ooi O, Davidoff D, et al. Abstract number 62: Cyproterone vs spironolactone as anti‐androgen therapy for transgender females receiving oestradiol therapy. Clin Endocrinol (Oxf) 2018; 89 (Suppl S1): 14.
- 41. Asscheman H, Gooren LJ, Eklund PL. Mortality and morbidity in transsexual patients with cross‐gender hormone treatment. Metabolism 1989; 38: 869–873.
- 42. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 2004; 291: 1701–1712.
- 43. Pinkerton JV. What are the concerns about custom‐compounded “bioidentical” hormone therapy? Menopause 2014; 21: 1298–1300.
- 44. Fagart J, Hillisch A, Huyet J, et al. A new mode of mineralocorticoid receptor antagonism by a potent and selective nonsteroidal molecule. J Biol Chem 2010; 285: 29932–29940.
- 45. Honer C, Nam K, Fink C, et al. Glucocorticoid receptor antagonism by cyproterone acetate and RU486. Mol Pharmacol 2003; 63: 1012–1020.
- 46. Gil M, Oliva B, Timoner J, et al. Risk of meningioma among users of high doses of cyproterone acetate as compared with the general population: evidence from a population‐based cohort study. Br J Clin Pharmacol 2011; 72: 965–968.
- 47. de Blok CJM, Klaver M, Wiepjes CM, et al. Breast development in transwomen after 1 year of cross‐sex hormone therapy: results of a prospective multicenter study. J Clin Endocrinol Metab 2018; 103: 532–538.
- 48. Bultynck C, Pas C, Defreyne J, et al. Self‐perception of voice in transgender persons during cross‐sex hormone therapy. Laryngoscope 2017; 127: 2796–2804.
- 49. Gao Y, Maurer T, Mirmirani P. Understanding and addressing hair disorders in transgender individuals. Am J Clin Dermatol 2018; 19: 517–527.
- 50. Ahmad S, Leinung M. The response of the menstrual cycle to initiation of hormonal therapy in transgender men. Transgend Health 2017; 2: 176–179.
- 51. Wierckx K, Van de Peer F, Verhaeghe E, et al. Short‐ and long‐term clinical skin effects of testosterone treatment in trans men. J Sex Med 2014; 11: 222–229.





Abstract
Introduction: Rising demand for gender‐affirming hormone therapy mandates a need for more formalised care of transgender and gender diverse (TGD) individuals in Australia. Estimates suggest that 0.1–2.0% of the population are TGD, yet medical education in transgender health is lacking. We aim to provide general practitioners, physicians and other medical professionals with specific Australian recommendations for the hormonal and related management of adult TGD individuals.
Main recommendations:
Changes in management as result of this position statement: Gender‐affirming hormone therapy is effective and, in the short term, relatively safe with appropriate monitoring. Further research is needed to guide clinical care and understand long term effects of hormonal therapies. We provide the first guidelines for medical practitioners to aid the provision of gender‐affirming care for Australian adult TGD individuals.