Polycystic ovary syndrome (PCOS) has recently been shown to affect a striking 12%–21% of Australian reproductive-age women, being more common among those who are overweight or of Indigenous background.1 PCOS can be a frustrating experience for women, a complex syndrome for clinicians and a scientific challenge for researchers, and is a major public health concern.
Although reproductive features are prominent, PCOS has potential for major metabolic consequences, including obesity and related type 2 diabetes mellitus (DM2) as well as cardiovascular disease (CVD), all of which are currently national health priority areas.2,3 It also has significant mental health and psychological impact, impairing quality of life (QoL).4,5 Because increased obesity exacerbates incidence, prevalence and severity of PCOS, and weight loss improves reproductive, metabolic and psychological features, lifestyle change should be first-line therapy for PCOS.6
It is estimated that 70% of Australian women with PCOS remain undiagnosed;1 clinical practice is inconsistent;7 psychological issues are under-recognised;5 and there is little focus on lifestyle and prevention, with most services targeting infertility and costly assisted reproductive technology. Given the prevalence, disease burden, health costs and clear gaps in care, PCOS is highlighted in national policy and has been prioritised by government, with funding for development of a national PCOS evidence-based guideline and translation of evidence into practice. Here, we present a brief general clinical introduction to PCOS, concise guideline algorithms and clinical pathways and a comprehensive summary of the Evidence based guideline for assessment and management of PCOS (available at http://www.managingpcos.org.au/pcos-evidence-based-guidelines).8
PCOS has significant and diverse implications, including reproductive (hyperandrogenism, hirsutism, anovulation, infertility), metabolic (insulin resistance [IR], impaired glucose tolerance, DM2, adverse cardiovascular risk profiles) and psychological features (increased anxiety and depression and worsened QoL).9 Presentation varies across the life span. Hyperandrogenic features are often most prominent among adolescents,10 fertility issues most prominent among women in their 20s and 30s, and metabolic challenges most notable after this.11 The propensity to weight gain and psychological challenges affect all ages, and metabolic features can occur early, especially among those who are overweight. Variations across ethnic groups should also be noted, such as fewer hyperandrogenic dermatological features and more severe metabolic features in Asian women, even without weight gain. Indigenous women appear to have a higher prevalence and severity of PCOS.1,12 The clinical presentation of PCOS is outlined in Figure 1.
Diagnosis of PCOS is now largely based on the Rotterdam criteria,14 which are inclusive of the original National Institutes of Health (NIH) criteria15 and require two of three key features: oligo- or anovulation, clinical and/or biochemical hyperandrogenism and polycystic ovaries on ultrasound (Figure 2). However, as noted, PCOS phenotypes vary widely depending on life stage, genotype, ethnicity and environmental factors, including lifestyle and body weight.
Diagnostic investigations must exclude other causes and include thyroid function tests and prolactin and follicle-stimulating hormone (FSH) levels.9 For diagnosis, androgen levels should be measured; however, optimal methodology remains very controversial and is addressed in Section 1. Vaginal ultrasound is often needed for diagnosis where hyperandrogenism and anovulation are not both clearly present. Ultrasound can check for polycystic ovaries and endometrial thickness. However, vaginal ultrasound should be reserved for sexually active women. The role of ultrasound remains controversial for adolescents, among whom a polycystic appearance of the ovaries is very common, potentially leading to overdiagnosis;16 hence, this area is also covered in the guideline. Other diagnostic investigations are based on clinical discretion.
PCOS is an endocrine disorder, the pathophysiology of which remains unclear. Genetic and environmental contributors combine with obesity, ovarian dysfunction and hormonal drivers to contribute to the aetiology of PCOS.17,18 The underlying hormonal imbalance may include a combination of increased androgens and/or hyperinsulinaemia secondary to IR (Figure 1). Greater understanding of cause has been hampered by a lack of ideal methods to assess either hyperandrogenism or IR. Hyperandrogenism is detected in around 60%–80% of women with PCOS, and IR is a pathophysiological contributor in around 50%–80%.19 Obesity increases reproductive features — hyperandrogenism, hirsutism, infertility and pregnancy complications — both independently and by exacerbating PCOS.20,21 Furthermore, obesity exacerbates the PCOS-related increased risk factors for impaired glucose tolerance, DM2 and CVD,22 while obesity also affects psychological features of PCOS.
PCOS is a chronic condition that manifests across the life course. Women with PCOS present with psychological,5,23 reproductive24 and metabolic implications. In terms of psychosocial implications, challenges to feminine identity and body image due to obesity, acne, excess hair, infertility and long-term health-related concerns compromise QoL and adversely affect mood and psychological wellbeing. With a higher prevalence and greater severity of depression and anxiety, low self-esteem, negative body image, and psychosexual dysfunction,5,25 assessment of psychological functioning in women with PCOS is vital. This is relevant to clinical care as mood disturbance, in turn, impairs QoL and adversely affects ability to self-manage and optimise lifestyle. Optimal approaches to screening and assessment of psychological functioning in PCOS are unknown and recognition is generally poor; hence, this area was prioritised in the guideline (Section 4). If mood disturbance is detected during screening, further assessment and management is required.
Reproductive and reproductive hormonal features are often the best-recognised features in PCOS as they form the basis of the diagnostic criteria.14 These include clinical and biochemical hyperandrogenism, anovulation, subfertility and polycystic ovaries on ultrasound. A key point is that fertility is not necessarily impaired in all PCOS cases — some women conceive without medical intervention, depending on the severity of the condition. Age and BMI have a critical role in infertility risk in PCOS; therefore, early family initiation (before the age of 30–35 years) combined with maintaining a BMI < 31 kg/m2 is ideal for increasing the chance of conceiving.
Metabolic features of PCOS include an apparent propensity for excess weight gain, an increased prevalence of prediabetes and DM2, a 5–10-fold risk of progression from prediabetes to DM2 and a 4–7-fold risk of DM2.11 Cardiovascular risk factors are increased and CVD appears more prevalent among women with PCOS despite inadequate long-term studies to appropriately address this question.22 In the general population, IR is a predictor of CVD.26,27 Women with PCOS also have an increased prevalence of metabolic syndrome (associated with an increased risk for DM2 and CVD),28 individual risk factors for CVD and clinical signs of atherosclerosis,29,30 which are all exacerbated by obesity. Women with PCOS are therefore a population at high risk of developing DM2 and CVD. As DM2 and subsequent CVD are the primary cause of death in Australian women, any increase in prevalence will have significant public health implications. It is also important to note that relatives of women with PCOS may have increased risk of diabetes and increased CVD risk factors. Metabolic features are often poorly appreciated in PCOS; hence, recommendations on screening and assessment of DM2 and CVD risk factors are covered in the guideline.
Obesity or excess weight is a major cause of chronic disease in Western countries. In Australia, 56% of the adult population is overweight (BMI ≥ 25 kg/m2) or obese (BMI ≥ 30 kg/m2). In 2007, 31% of women were overweight and 24% of women were obese. Recent data from the Australian Longitudinal Study on Women’s Health showed that among 26–31-year-old women, 20.4% were overweight and a further 13.9% were obese.31 Overall, the proportion of adults who are obese has doubled in the past 20 years.32 Obesity is now the primary cause of chronic disease among Australian women, with adverse outcomes including DM2 and CVD.31 Obesity has a specific impact on women’s reproductive health, increasing the prevalence and severity of PCOS, infertility, pregnancy complications, gestational diabetes and fetal pregnancy complications, with substantial and escalating economic costs.33,34 Indeed, the adverse impact of obesity on fertility, exacerbated by delay in childbearing, is resulting in a significant social, health and economic burden in Australia.35 Given the dramatic increase in obesity, the guideline addresses weight loss and prevention of weight gain through lifestyle intervention (Section 5).
PCOS management should focus on support and education, and needs to strongly emphasise healthy lifestyle, with targeted medical therapy as required. Given the putative aetiological role of IR and obesity in PCOS, prevention of weight gain is important across the life span. Furthermore, among those who are already overweight, multidisciplinary lifestyle intervention aimed at improving IR and aiding weight management is recognised as first-line therapy for most women who are overweight.6 Modest weight loss of 5%–10% of initial body weight significantly reduces IR and has been demonstrated to ameliorate many of the features of PCOS.6 Optimal methods for achieving weight loss and prevention of weight gain remain unclear and are a focus of this guideline (Section 5).
In addition to lifestyle measures, therapy in PCOS can be targeted to specific clinical presentations. Although there is a plethora of options for therapy in PCOS, in this guideline we have focused on the most controversial interventions, where little guidance is currently available. Assessment of mood disorders and emotional wellbeing is prioritised in the guideline, yet treatment is well guided by a range of existing clinical guidance tools.36-43 Hirsutism treatment is also guided by a recent and comprehensive international statement,44 so these areas are not covered in the guideline. Infertility remains a highly controversial area and is covered in detail in the guideline (Sections 6, 7 and 8). DM2 and CVD risk assessment is included (Section 3); yet treatments for these established complications are covered in other specific national evidenced-based guidelines,45,46 and have not been reproduced here. Optimal therapy for infertility is one of the most controversial areas of PCOS management and includes lifestyle interventions, medical and surgical ovulation induction, consideration of bariatric surgery for preconception weight loss, and in-vitro fertilisation (IVF). All these areas are covered in the guideline except for IVF therapy, as this was deemed to be of a lower priority than the first-line lifestyle measures and ovulation induction therapies. Potential targeted treatment options for PCOS are summarised in Box 1.
Box 1. Summary of potential targeted treatment options for polycystic ovary syndrome (PCOS)
Lifestyle change (5%–10% weight loss + structured exercise)
Oral contraceptive pill (OCP) (low oestrogen doses [eg, 20 μg] may have less impact on insulin resistance)47
Cyclic progestins (eg, 10 mg medroxyprogesterone acetate 10–14 days every 2–3 months)
Metformin (improves ovulation and menstral cyclicity)
Choice of options depends on patient preferences; impact on wellbeing; and access and affordability:44
Self-administered and professional cosmetic therapy are first line (laser recommended)
Eflornithine cream can be added and may induce a more rapid response
If cosmetic therapy is not adequate, pharmacological therapy can be considered
Pharmacological therapy
Medical therapy if patient is concerned and cosmetic therapy is ineffective/inaccessible/unaffordable
Primary therapy is the OCP (monitor glucose tolerance in those at risk of diabetes)
Anti-androgen monotherapy (eg, spironolactone or cyproterone acetate) should not be used without adequate contraception
Trial therapies for ≥ 6 months before changing dose or medication
Combination therapy — if ≥ 6 months of OCP is ineffective, add anti-androgen to OCP (twice daily spironolactone > 50 mg or cyproterone acetate 25 mg/day, days 1–10 of OCP)
Lifestyle intervention (to optimise preconception health and fertility and reduce pregnancy and long-term complications)
Advise on folate, smoking cessation and optimal weight and exercise before conception
Given age-related infertility, advise women to optimise family initiation
Infertility therapies may include clomiphene citrate, metformin, gonadotrophins, surgery and in-vitro fertilisation.
Lifestyle change: > 5% weight loss in those who are overweight reduces diabetes risk by approximately 50%–60% in high-risk groups48
Optimise cardiovascular risk factors
Adapted and reproduced with permission from Teede et al,9 not generated directly from the evidence-based guidelines. Hirsutism therapy is summarised from existing hirsutism clinical practice guidelines.44 * Metformin and the OCP are not currently approved for use to manage PCOS by many regulatory bodies. The OCP is indicated for contraception and metformin for diabetes. However, their use is supported by evidence and is recommended by international and national specialist societies.49
The prevalence of PCOS among Indigenous Australian women appears to be as high as 21% by the Rotterdam criteria12 and the NIH criteria,50 and increases with rising BMI.12 In a group of Indigenous women with PCOS, 30.3% were obese and 7.0% had a normal BMI.12
DM2 and obesity are associated with major morbidity among Indigenous women. The National Aboriginal and Torres Strait Islander Health Survey (NATSIHS) found that Indigenous Australians are 1.2 times more likely to be overweight or obese than non-Indigenous Australians, and this disparity is greatest for women.51 DM2 is the second-commonest cause of mortality and disability-adjusted life-years (DALY) among Indigenous women.52 The DALY rate ratios (age-standardised to total Indigenous population) for ischaemic heart disease and DM2 among Indigenous women, compared with all Australian women, are 6.6 and 6.3, respectively; and the mortality rate ratio is 5.0 for ischaemic heart disease and 18.9 for DM2.52
The leading cause of burden of disease among Indigenous women in the NATSIHS was anxiety and depression, accounting for 10% of the burden.51 Little is known about the prevalence of eating disorders and disordered eating among Indigenous women. Social and cultural factors influence emotional wellbeing, and the challenges facing many Indigenous women are likely to amplify the impact of PCOS on emotional wellbeing. Further research in this area is needed.
Currently, there is limited consensus among different medical specialties as to the optimal management of PCOS in Australia.7,53 Diagnosis and treatment of PCOS can therefore differ depending on the health professional consulted (eg, general practitioner, endocrinologist or gynaecologist).7 There are limited clinical guidelines and no evidence-based guidelines, either in Australia or internationally, for assessment or management of women with PCOS; rather, PCOS is briefly mentioned within guidelines for the management of obesity and DM2.45,54 Where international clinical guidelines for the assessment and management of women with PCOS exist, they are informed by expertise and, in some cases, evidence, but are not rigorously developed evidenced-based guidelines; they do not consider psychological issues; and they offer simplistic advice on lifestyle management of PCOS. There is no guidance on the assessment and management of PCOS among Indigenous women, nor any adaptation for the Australian context.
Overall, many areas of controversy remain in PCOS. Comprehensive evidence-based guidelines are warranted to optimise diagnosis, assessment and management. Accordingly, the Jean Hailes Foundation for Women’s Health has facilitated the formation of an independent PCOS Australian Alliance, bringing together health professionals, researchers, consumers and policymakers to advance knowledge and quality of care in PCOS. The federal government has funded the PCOS Australian Alliance, under the auspices of the Jean Hailes Foundation, to produce national evidence-based guidelines. The full version of the guideline has been approved by the National Health and Medical Research Council (NHMRC) (for detail about obtaining NHMRC approval, please see the full guideline), is endorsed by the Royal Australian College of General Practitioners and is freely available at http://www.managingpcos.org.au/pcos-evidence-based-guidelines.8 This is a summary version of the full guideline. The Jean Hailes Foundation was funded to translate the guideline into practice, including freely available independent evidence-based information on PCOS for health professionals and women at http://www.managingpcos.org.au.
PCOS is a syndrome, and as such, no single diagnostic criterion is sufficient for diagnosis. The 2003 Rotterdam consensus workshop concluded that PCOS diagnosis requires at least two of: oligo- or anovulation, hyperandrogenism (clinical and/or biochemical) and polycystic ovaries on ultrasound (Figure 2).14 The evidence-based guideline development groups and the Alliance agreed to endorse the Rotterdam diagnostic criteria for the guideline, while recognising there are current limitations of all definitions.
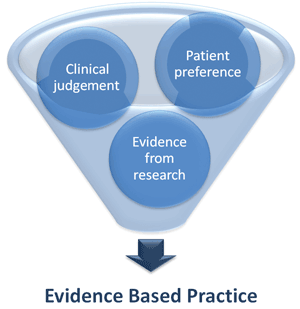
Guidelines are intended to improve patient outcomes, promote standardised care, develop standards to assess the clinical practice of health care professionals, and promote research and translation into practice. Guidelines are developed by drawing from clinician judgement, patient preference and research evidence, and are intended to aid clinical judgement and patient preference, not to replace it (Figure 3). The ultimate decision about clinical management of an individual patient will always depend on the clinical circumstances, patient preferences, and the clinical judgement of the health care team. Although there are many types of guidelines, this NHMRC-approved evidence-based guideline followed a rigorous, systematic process of development, which is briefly outlined below and in detail in the full guideline.8
Figure 3. Evidence-based guidelines are intended as an aid to clinical judgement and patient preference, not to replace it
This guideline was developed as outlined in the NHMRC standards and procedures for externally developed guidelines.55
These multidisciplinary groups determined and prioritised the clinical questions addressed in this guideline and developed the clinical practice and research recommendations from the evidence reviews. For more detail about the development and prioritisation of clinical questions, please see the full guideline.8
Evidence reviews were conducted for each of the 22 identified clinical questions. Search strategies were developed according to a-priori selection criteria for each clinical question. Searches were limited to English language articles and there were no limits on year of publication. The literature was searched until November 2010. The following electronic databases were employed to identify relevant evidence: Australasian Medical Index, CINAHL, the Cochrane Library, the Cochrane Database of Systematic Reviews, DARE (Database of Abstracts of Reviews of Effects), the Cochrane Central Register of Controlled Trials, the Cochrane Database of Methodology Reviews, the Cochrane Methodology Register, Health Technology Assessment Database, the United Kingdom National Health Service Economic Evaluation Database, EMBASE, EBMR, MEDLINE and PsycINFO. Bibliographies of relevant studies identified by the search strategy and relevant reviews/meta-analyses were also searched. Included studies were classified according to the NHMRC levels of evidence56 and appraised using a-priori criteria according to study design, using a descriptive component approach to assign a risk of bias rating.57 In accordance with the selection criteria, data were extracted from included studies using a specially developed data extraction form,57 and meta-analyses were performed where appropriate. The guideline development groups were able to develop guideline recommendations from these evidence reviews. The evidence reviews for each question can be found in the supporting document to the full guideline: Evidence report: evidence based guidelines for assessment and management of PCOS, available at http://www.managingpcos.org.au/pcos-evidence-based-guidelines.
Each evidence-based recommendation was given an overall grading from A to D, according to the NHMRC grades of recommendations for guideline developers (Table 1).56 Evidence grading is provided primarily to inform users about the strength of the evidence underpinning each recommendation. Where there was insufficient high-quality evidence in specific patient groups, lower-quality evidence or data from other patient groups, and where there was consensus among the guideline development group, combined with clinician and patient preferences, clinical consensus recommendations were developed. Clinical practice points have also been included, where important issues (such as safety, side effects or risks) arose from discussion of evidence-based or clinical consensus recommendations. Further points of relevance to the clinical implementation of recommendations were made in “implications of the recommendations” sections, including consideration of resource implications.
Table 1. National Health and Medical Research Council grades for recommendations56
Body of evidence can be trusted to guide practice in most situations. |
|
Body of evidence is weak and recommendation must be applied with caution. |
Public and targeted consultation on the draft guideline was conducted for 30 days commencing 5 March 2011, in accordance with the legislative requirements for approval of externally developed guidelines under Section 14A of the National Health and Medical Research Council Act 1992 (Cwlth). All aspects of the guideline were developed as outlined in the NHMRC standards and procedures for externally developed guidelines,55 and accordingly, the guideline was approved by the NHMRC in July 2011. In approving the full version of the guideline, the NHMRC is satisfied that it is based on the systematic identification and synthesis of the best available scientific evidence and makes clear recommendations for health professionals practising in an Australian health care setting.
This guideline does not seek to provide full safety and usage information on pharmacological and surgical interventions. The pharmacological and surgical interventions recommended in the guideline should not be applied without consideration of the patient’s clinical profile and personal preferences. It is recommended that the reader consults the Therapeutic Guidelines (http://www.tg.com.au) and the National Prescribing Service (http://www.nps.org.au) for detailed prescribing information, including indications, drug dosages, methods and routes of administration, contraindications, supervision and monitoring, product characteristics, and adverse effects.
It is intended that this evidence-based guideline summary be used alongside the full guideline.8 The guideline should be considered according to the limitations outlined within, and used in conjunction with clinical judgement and patient preference. For a detailed description of the methodology used to develop the guideline, please see the full guideline.8
- 1. March W, Moore V, Willson K, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 2010; 25: 544-551.
- 2. Moran L, Teede H. Metabolic features of the reproductive phenotypes of polycystic ovary syndrome. Hum Reprod Update 2009; 15: 477-488.
- 3. Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet 2007; 370: 685-697.
- 4. Ching H, Burke V, Stuckey B. Quality of life and psychological morbidity in women with polycystic ovary syndrome: body mass index, age and the provision of patient information are significant modifiers. Clin Endocrinol (Oxf) 2007; 66: 373-379.
- 5. Deeks A, Gibson-Helm M, Teede H. Anxiety and depression in polycystic ovary syndrome: a comprehensive investigation. Fertil Steril 2010; 93: 2421-2423.
- 6. Moran L, Pasquali R, Teede H, et al. Treatment of obesity in polycystic ovary syndrome: a position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertil Steril 2009; 92: 1966-1982.
- 7. Cussons A, Stuckey B, Walsh J, et al. Polycystic ovarian syndrome: marked differences between endocrinologists and gynaecologists in diagnosis and management. Clin Endocrinol (Oxf) 2005; 62: 289-295.
- 8. Evidence-based guideline for the assessment and management of polycystic ovary syndrome. Melbourne: Jean Hailes Foundation for Women’s Health on behalf of the PCOS Australian Alliance, 2011.
- 9. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med 2010; 8: 41.
- 10. Moran L, Gibson-Helm M, Teede H, Deeks A. Polycystic ovary syndrome: a biopsychosocial understanding in young women to improve knowledge and treatment options. J Psychosom Obstet Gynaecol 2010; 31: 24-31.
- 11. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2010; 16: 347-363.
- 12. Boyle J. Polycystic ovary syndrome and associated metabolic features in Indigenous women in the Northern Territory [PhD thesis]. Adelaide: University of Adelaide, 2011.
- 13. Teede H, Zoungas S, Deeks A, et al. Check: Independent learning program for GPs. Polycystic ovary syndrome. Melbourne: Royal Australian College of General Practitioners, 2008.
- 14. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004; 81: 19-25.
- 15. Zawadaki R, Dockerty M. Diagnostic criteria for polycystic ovarian syndrome: towards a rational approach. In: Dunaif A, Given JR, Haseltine F, Merriam GR, editors. Current issues in endocrinology and metabolism: polycystic ovary syndrome. Boston: Blackwell Scientific, 1992: 377-384.
- 16. Kristensen S, Ramlau-Hansen C, Ernst E, et al. A very large proportion of young Danish women have polycystic ovaries: is a revision of the Rotterdam criteria needed? Hum Reprod 2010; 25: 3117-3122.
- 17. Legro R, Strauss J. Molecular progress in infertility: polycystic ovary syndrome. Fertil Steril 2002; 78: 569-576.
- 18. Doi S, Al-Zaid M, Towers P, et al. Ovarian steroids modulate neuroendocrine dysfunction in polycystic ovary syndrome. J Endocrinol Invest 2005; 28: 882-892.
- 19. Legro R, Castracane V, Kauffman R. Detecting insulin resistance in polycystic ovary syndrome: purposes and pitfalls. Obstet Gynecol Surv 2004; 59: 141-154.
- 20. Balen A, Conway G, Kaltsas G, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod 1995; 10: 2107-2111.
- 21. Kiddy D, Sharp P, White D, et al. Differences in clinical and endocrine features between obese and non-obese subjects with polycystic ovary syndrome: an analysis of 263 consecutive cases. Clin Endocrinol (Oxf) 1990; 32: 213-220.
- 22. Shaw L, Bairey Merz C, Azziz R, et al. Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health – National Heart, Lung, and Blood Institute sponsored Women’s Ischemia Syndrome Evaluation. J Clin Endocrinol Metab 2008; 93: 1276-1284.
- 23. Himelein M, Thatcher S. Polycystic ovary syndrome and mental health: a review. Obstet Gynecol Surv 2006; 61: 723-732.
- 24. Boomsma C, Eijkemans M, Hughes E, et al. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update 2006; 12: 673-683.
- 25. Coffey S, Mason H. The effect of polycystic ovary syndrome on health-related quality of life. Gynecol Endocrinol 2003; 17: 379-386.
- 26. Haffner S. The insulin resistance syndrome revisited. Diabetes Care 1996; 19: 275.
- 27. Koskinen P, Manttari M, Manninen V. Coronary heart disease incidence in NIDDM patients in the Helsinki Heart study. Diabetes Care 1992; 15: 820.
- 28. Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2005; 90: 1929-1935.
- 29. Meyer C, McGrath B, Cameron J, et al. Vascular dysfunction and metabolic parameters in polycystic ovary syndrome. J Clin Endocrinol Metab 2005; 90: 4630-4635.
- 30. Meyer C, McGrath B, Teede H. Overweight women with polycystic ovary syndrome have evidence of subclinical cardiovascular disease. J Clin Endocrinol Metab 2005; 90: 5711-5716.
- 31. Women’s Health Australia. The Australian Longitudinal Study on Women’s Health. Australian women and their weight — a growing problem. Newcastle: WHA, 2005. http://www.alswh.org.au/Reports/Achievements/achievements-weight.pdf (accessed Nov 2010).
- 32. Australian Institute of Health and Welfare. Australia’s health 2006: the tenth biannual health report of the Australian Institute of Health and Welfare. Canberra: AIHW, 2006. (AIHW Cat. No. AUS 73.)
- 33. Finkelstein E, Fiebelkorn I, Wang G. National medical spending attributable to overweight and obesity: how much and who’s paying? Health Aff (Millwood) 2003; Suppl Web Exclusives: W3-219-W3-226.
- 34. Wolf AM. Economic outcomes of the obese patient. Obes Res 2002; 10 Suppl 1: 58S-62S.
- 35. Hutchison S, Zoungas S, Teede H. Insulin levels, insulin resistance and the use of metformin in polycystic ovary syndrome [letter]. Med J Aust 2007; 186: 268-269. <MJA full text>
- 36. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Text revision. Arlington, Va: American Psychiatric Publishing Inc, 2000.
- 37. Australian Medical Association. Body image and health — 2002. Revised 2009 [position statement]. Canberra: AMA, 2009.
- 38. beyondblue. Clinical practice guidelines: depression in adolescents and young adults. Melbourne: beyondblue, 2011. http://www.beyondblue.org.au/index.aspx?link_id=6.1247 (accessed Aug 2011).
- 39. National Collaborating Centre for Mental Health. Obsessive–compulsive disorder: core interventions in the treatment of obsessive–compulsive disorder and body dysmorphic disorder. London: National Institute for Health and Clinical Excellence, British Psychological Society, Royal College of Psychiatrists, 2006. (National Clinical Practice Guideline No. 31.)
- 40. National Institute for Health and Clinical Excellence. Depression in adults with a chronic physical health problem: treatment and management. London: National Collaborating Centre for Mental Health, National Health Service, 2009.
- 41. National Institute for Clinical Excellence. Eating disorders: core interventions in the treatment and management of anorexia nervosa, bulimia nervosa and related eating disorders. London: NICE, 2004.
- 42. National Institute for Health and Clinical Excellence. Depression: the treatment and management of depression in adults. London: National Collaborating Centre for Mental Health, National Health Service, 2009.
- 43. National Institute for Health and Clinical Excellence. Management of generalised anxiety disorder and panic disorder (with or without agoraphobia) in adults: management in primary, secondary and community care. London: National Collaborating Centre for Mental Health, National Collaborating Centre for Primary Care, National Health Service, 2011.
- 44. Martin KA, Chang RJ, Ehrmann DA, et al. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2008; 93: 1105-1120.
- 45. National Health and Medical Research Council. National evidence based guidelines for the management of type 2 diabetes mellitus. Canberra: NHMRC, 2001.
- 46. National Vascular Disease Prevention Alliance. Guidelines for the assessment of absolute cardiovascular disease risk. Canberra: National Heart Foundation of Australia, 2009.
- 47. Meyer C, McGrath B, Teede H. Effects of medical therapy on insulin resistance and the cardiovascular system in polycystic ovary syndrome. Diabetes Care 2007; 30: 471-478.
- 48. Knowler W, Barrett-Connor E, Fowler S, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393-403.
- 49. Teede H, Hutchison S, Zoungas S. The management of insulin resistance in polycystic ovary syndrome. Trends Endocrinol Metab 2007; 18: 273-279.
- 50. Davis S, Knight S, White V, et al. Preliminary indication of a high prevalence of polycystic ovary syndrome in Indigenous Australian women. Gynecol Endocrinol 2002; 16: 443-446.
- 51. Australian Bureau of Statistics. National and Torres Strait Islander health survey 2004–05. Canberra: ABS, 2005. (ABS Cat. No. 4715.0.)
- 52. Vos T, Barker B, Stanley L, Lopez AD. The burden of disease and injury in Aboriginal and Torres Strait Islander peoples 2003. Brisbane: Centre for Burden of Disease and Cost-Effectiveness, School of Population Health, University of Queensland, 2007.
- 53. Azziz R, Carmina E, Dewailly E, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 2009; 91: 456-457.
- 54. National Health and Medical Research Council. Clinical practice guidelines for the management of overweight and obesity in adults. Canberra: Australian Government Publishing Service, 2004. http://www.health.gov.au/internet/main/publishing.nsf/Content/obesityguidelines-guidelines-adults.htm (accessed Aug 2011).
- 55. National Health and Medical Research Council. NHMRC standards and procedures for externally developed guidelines. Canberra: NHMRC, 2007.
- 56. National Health and Medical Research Council. NHMRC levels of evidence and grades for recommendations for developers of guidelines. Canberra: NHMRC, 2009.
- 57. Southern Health Centre for Clinical Effectiveness. Critical appraisal templates. Melbourne: Southern Health, 2010.
- 58. Davison S, Bell R, Donath S, et al. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab 2005; 90: 3847-3853.
- 59. Vermeulen A, Verdonck L, Kaufman J. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999; 84: 3666-3672.
- 60. Escobar-Morreale HF, Asunción M, Calvo RM, et al. Receiver operating characteristic analysis of the performance of basal serum hormone profiles for the diagnosis of polycystic ovary syndrome in epidemiological studies. Eur J Endocrinol 2001; 145: 619-624.
- 61. Hahn S, Kuehnel W, Tan S, et al. Diagnostic value of calculated testosterone indices in the assessment of polycystic ovary syndrome. Clin Chem Lab Med 2007; 45: 202-207.
- 62. Koskinen P, Penttilä TA, Anttila L, et al. Optimal use of hormone determinations in the biochemical diagnosis of the polycystic ovary syndrome. Fertil Steril 1996; 65: 517-522.
- 63. Lemarchand-Béraud T, Zufferey M, Reymond M, Rey I. Maturation of the hypothalamo–pituitary–ovarian axis in adolescent girls. J Clin Endocrinol Metab 1982; 54: 241-246.
- 64. Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil 1967; 12: 77-126.
- 65. Flug D, Largo R, Prader A. Menstrual patterns in adolescent Swiss girls: a longitudinal study. Ann Hum Biol 1984; 11: 495-508.
- 66. Widholm O, Kantero R. A statistical analysis of the menstrual patterns of 8,000 Finnish girls and their mothers. Acta Obstet Gynecol Scand Suppl 1971; 14 Suppl 14: 1-36.
- 67. Adams Hillard P. Menstruation in young girls: a clinical perspective. Obstet Gynecol 2002; 99: 655-662.
- 68. Slap G. Menstrual disorders in adolescence. Best Pract Res Clin Obstet Gynaecol 2003; 17: 75-92.
- 69. Apter D, Vihko R. Early menarche, a risk factor for breast cancer, indicates early onset of ovulatory cycles. J Clin Endocrinol Metab 1983; 57: 82-86.
- 70. Hart R, Sloboda D, Doherty D, et al. Prenatal determinants of uterine volume and ovarian reserve in adolescence. J Clin Endocrinol Metab 2009; 94: 4931-4937.
- 71. Blank SK, Helm KD, McCartney CR, Marshall JC. Polycystic ovary syndrome in adolescence. Ann N Y Acad Sci 2008; 1135: 76-84.
- 72. Mortensen M, Rosenfield R, Littlejohn E. Functional significance of polycystic-size ovaries in healthy adolescents. J Clin Endocrinol Metab 2006; 91: 3786-3790.
- 73. Chen Y, Yang D, Li L, Chen X. The role of ovarian volume as a diagnostic criterion for Chinese adolescents with polycystic ovary syndrome. J Pediatr Adolesc Gynecol 2008; 21: 347-350.
- 74. San Martín-Rodríguez L, Beaulieu MD, D’Amour D, Ferrada-Videla M. The determinants of successful collaboration: a review of theoretical and empirical studies. J Interprof Care 2005; 19 Suppl 1: 132-147.
- 75. Smith G, Clarke D. Assessing the effectiveness of integrated interventions: terminology and approach. Med Clin North Am 2006; 90: 533-548.
- 76. Smith S, Allwright S, O’Dowd T. Effectiveness of shared care across the interface between primary and specialty care in chronic disease management. Cochrane Database Syst Rev 2007; (3): CD004910.
- 77. National Heart Foundation of Australia; Cardiac Society of Australia and New Zealand. Lipid management guidelines 2001 — summary paper. Med J Aust 2001; 175 (9 Suppl): S57-S88.
- 78. Wild R, Carmina E, Diamanti-Kandarakis E, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab 2010; 95: 2038-2049.
- 79. Tonkin A, Barter P, Best J, et al; National Heart Foundation of Australia; Cardiac Society of Australia and New Zealand. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: position statement of lipid management. Heart Lung Circ 2005; 14: 275-291.
- 80. National Blood Pressure and Vascular Disease Advisory Committee. Guide to management of hypertension 2008. Updated 2010. Canberra: National Heart Foundation of Australia, 2010.
- 81. Cussons A, Watts G, Burke V, et al. Cardiometabolic risk in polycystic ovary syndrome: a comparison of different approaches to defining the metabolic syndrome. Hum Reprod 2008; 23: 2352-2358.
- 82. Chan D, Watts G. Dyslipidaemia in the metabolic syndrome and type 2 diabetes: pathogenesis, priorities, pharmacotherapies. Expert Opin Pharmacother 2011; 12: 13-30.
- 83. Moran L, Hutchison S, Meyer C, et al. A comprehensive assessment of endothelial function in overweight women with and without polycystic ovary syndrome. Clin Sci (Lond) 2009; 116: 761-770.
- 84. Ehrmann D, Liljenquist D, Kasza K, et al. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2006; 91: 48-53.
- 85. Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 1999; 84: 165-168.
- 86. Legro R, Kunselman A, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med 2001; 111: 607-613.
- 87. Birdsall M, Farquhar C, White H. Association between polycystic ovaries and extent of coronary artery disease in women having cardiac catheterization. Ann Intern Med 1997; 126: 32-35.
- 88. Talbott E, Guzick D, Sutton-Tyrrell K, et al. Evidence for the association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol 2000; 20: 2414-2421.
- 89. Wild S, Pierpoint T, McKeigue P, Jacobs H. Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a retrospective cohort study. Clin Endocrinol (Oxf) 2000; 52: 595-600.
- 90. Solomon C. The epidemiology of polycystic ovary syndrome. Prevalence and associated disease risks. Endocrinol Metab Clin North Am 1999; 28: 247-263.
- 91. Gorgels W, v d Graaf Y, Blankenstein M, et al. Urinary sex hormone excretions in premenopausal women and coronary heart disease risk: a nested case-referent study in the DOM-cohort. J Clin Epidemiol 1997; 50: 275-281.
- 92. Pierpoint T, McKeigue P, Isaacs A, et al. Mortality of women with polycystic ovary syndrome at long term follow-up. J Clin Epidemiol 1998; 51: 581-586.
- 93. Rachon D, Teede H. Ovarian function and obesity — interrelationship, impact on women’s reproductive lifespan and treatment options. Mol Cell Endocrinol 2010; 316: 172-179.
- 94. Australian Bureau of Statistics. Causes of death. Canberra: ABS, 2010. (ABS Cat. No. 3303.0.) http://abs.gov.au/AUSSTATS/abs@.nsf/allprimarymain features/5CCE256209F55B25CA2578840012A040?opendocument (accessed Aug 2011).
- 95. World Health Organization. Preventing chronic diseases: a vital investment: WHO global report. Geneva: WHO, 2005.
- 96. Geiss L, Herman W, Smith P. Mortality in non-insulin-dependent diabetes. In: Harris MI, Cowie CC, Stern MP, et al; National Diabetes Data Group, editors. Diabetes in America. 2nd ed. Washington, DC: US Government Printing Office, 1995: 233-257.
- 97. Chen L, Magliano D, Balkau B, et al. AUSDRISK: an Australian Type 2 Diabetes Risk Assessment Tool based on demographic, lifestyle and simple anthropometric measures. Med J Aust 2010; 192: 197-202. <MJA full text>
- 98. Colagiuri S, Davies D, Girgis S, et al. National evidence based guideline for case detection and diagnosis of type 2 diabetes. Canberra: Diabetes Australia, NHMRC, 2009.
- 99. Teede H, Stuckey B. Polycystic ovary syndrome and abnormal glucose tolerance [editorial]. Med J Aust 2007; 187: 324-325. <MJA full text>
- 100. Reaven G. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr 2005; 25: 391-406.
- 101. DeUgarte C, Bartolucci C, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril 2005; 83: 1454-1460.
- 102. Acien P, Quereda F, Matallin P, et al. Insulin, androgens, and obesity in women with and without polycystic ovary syndrome: a heterogeneous group of disorders. Fertil Steril 1999; 72: 32-40.
- 103. Moran L, Strauss B, Teede H. Diabetes Risk Score in the diagnostic categories of polycystic ovary syndrome. Fertil Steril 2011; 95: 1742-1748.
- 104. Ehrmann D, Barnes R, Rosenfield R, et al. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 1999; 22: 141-146.
- 105. Norman R, Masters L, Milner C, et al. Relative risk of conversion from normoglycaemia to impaired glucose tolerance or non-insulin dependent diabetes mellitus in polycystic ovarian syndrome. Hum Reprod 2001; 16: 1995-1998.
- 106. Alberti K, Zimmet P, Shaw J. International Diabetes Federation: a consensus on type 2 diabetes prevention. Diabet Med 2007; 24: 451-463.
- 107. Azziz R, Carmina E, Dewailly D, et al. Position statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 2006; 91: 4237-4245.
- 108. Tomlinson J, Millward A, Stenhouse E, Pinkney J. Type 2 diabetes and cardiovascular disease in polycystic ovary syndrome: what are the risks and can they be reduced? Diabet Med 2010; 27: 498-515.
- 109. Bhattacharya S, Jha A. Prevalence and risk of depressive disorders in women with polycystic ovary syndrome (PCOS). Fertil Steril 2010; 94: 357-359.
- 110. Laggari V, Diareme S, Christogiorgos S, et al. Anxiety and depression in adolescents with polycystic ovary syndrome and Mayer–Rokitansky–Küster-Hauser syndrome. J Psychosom Obstet Gynaecol 2009; 30: 83-88.
- 111. Australian Bureau of Statistics. ABS National Survey of Mental Health Wellbeing: summary of results, 2007. Canberra: ABS, 2008. (ABS Cat. No. 4326.0.)
- 112. Gwynn RC, McQuistion HL, McVeigh KH, et al. Prevalence, diagnosis, and treatment of depression and generalized anxiety disorder in a diverse urban community. Psychiatr Serv 2008; 59: 641-647.
- 113. Benson S, Hahn S, Tan S, et al. Prevalence and implications of anxiety in polycystic ovary syndrome: results of an internet-based survey in Germany. Hum Reprod 2009; 24: 1446-1451.
- 114. Deeks A, Gibson-Helm M, Teede H. Is having polycystic ovary syndrome (PCOS) a predictor of poor psychological function including depression and anxiety? Hum Reprod 2011; 26: 1399-1407.
- 115. Deeks A, Gibson-Helm M, Teede H. Negative body image and lower self-efficacy in women with polycystic ovary syndrome. Proceedings of the Australian Society for Behavioural Health and Medicine 8th Annual Scientific Conference; 2010; Feb 10-12; Brisbane.
- 116. Dawber R. Guidance for the management of hirsutism. Curr Med Res Opin 2005; 21: 1227-1234.
- 117. Trent M, Austin SB, Rich M, Gordon CM. Overweight status of adolescent girls with polycystic ovary syndrome: body mass index as mediator of quality of life. Ambul Pediatr 2005; 5: 107-111.
- 118. Hay P, Mond J, Buttner P, Darby A. Eating disorder behaviors are increasing: findings from two sequential community surveys in South Australia. PLoS One 2008; 3: e1541.
- 119. Fairburn CG, Harrison PJ. Eating disorders. Lancet 2003; 361: 407-415.
- 120. McCluskey S, Lacey J, Pearce J. Binge-eating and polycystic ovaries. Lancet 1992; 340: 723.
- 121. Raphael F, Rodln D, Peattie A, et al. Ovarian morphology and insulin sensitivity in women with bulimia nervosa. Clin Endocrinol (Oxf) 1995; 43: 451-455.
- 122. Hirschberg AL, Naessén S, Stridsberg M, et al. Impaired cholecystokinin secretion and disturbed appetite regulation in women with polycystic ovary syndrome. Gynecol Endocrinol 2004; 19: 79-87.
- 123. Jahanfar S, Eden J, Nguyent T. Bulimia nervosa and polycystic ovary syndrome. Gynecol Endocrinol 1995; 9: 113-117.
- 124. Månsson M, Holte J, Landin-Wilhelmsen K, et al. Women with polycystic ovary syndrome are often depressed or anxious — a case control study. Psychoneuroendocrinology 2008; 33: 1132-1138.
- 125. Fairburn CG, Wilson G, editors. Binge eating: nature, assessment and treatment. New York: Guilford, 1993.
- 126. Hay PP, Bacaltchuk J, Stefano S, Kashyap P. Psychological treatments for bulimia nervosa and binging. Cochrane Database Syst Rev 2009; (4): CD000562.
- 127. Shapiro J, Berkman N, Brownley K, et al. Bulimia nervosa treatment: a systematic review of randomized controlled trials. Int J Eat Disord 2007; 40: 321-336.
- 128. Wilson G. Psychological treatment of eating disorders. Annu Rev Clin Psychol 2005; 1: 439-465.
- 129. Watson J, Davies T. ABC of mental health: psychosexual problems. BMJ 1997; 315: 239-242.
- 130. Elsenbruch S, Hahn S, Kowalsky D, et al. Quality of life, psychosocial well-being, and sexual satisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2003; 88: 5801-5807.
- 131. Hahn S, Benson S, Elsenbruch S, et al. Metformin treatment of polycystic ovary syndrome improves health-related quality-of-life, emotional distress and sexuality. Hum Reprod 2006; 21: 1925-1934.
- 132. Hahn S, Janssen O, Tan S, et al. Clinical and psychological correlates of quality-of-life in polycystic ovary syndrome. Eur J Endocrinol 2005; 153: 853-860.
- 133. Janssen O, Hahn S, Tan S, et al. Mood and sexual function in polycystic ovary syndrome. Semin Reprod Med 2008; 26: 45-52.
- 134. Drosdzol A, Skrzypulec V, Mazur B, Pawliñska-Chmara R. Quality of life and marital sexual satisfaction in women with polycystic ovary syndrome. Folia Histochem Cytobiol 2007; 45 Suppl 1: S93-S97.
- 135. Glueck C, Dharashivkar S, Wang P, et al. Obesity and extreme obesity, manifest by ages 20–24 years, continuing through 32–41 years in women, should alert physicians to the diagnostic likelihood of polycystic ovary syndrome as a reversible underlying endocrinopathy. Eur J Obstet Gynecol Reprod Biol 2005; 122: 206-212.
- 136. Teede H, Deeks A, Gibson-Helm M, et al. Body mass index as a predictor of polycystic ovary syndrome risk: results of a longitudinal cohort study [abstract no. 246]. Endocrine Society Annual Meeting; 2010 Jun 19-22; San Diego, Calif.
- 137. Clark AM, Thornley B, Tomlinson L, et al. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod 1998; 13: 1502-1505.
- 138. Huber-Buchholz M, Carey D, Norman R. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: role of insulin sensitivity and luteinizing hormone. J Clin Endocrinol Metab 1999; 84: 1470-1474.
- 139. Moran LJ, Noakes M, Clifton PM, et al. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab 2003; 88: 812-819.
- 140. Thomson RL, Buckley JD, Lim SS, et al. Lifestyle management improves quality of life and depression in overweight and obese women with polycystic ovary syndrome. Fertil Steril 2010; 94: 1812-1816.
- 141. Andersen P, Seljeflot I, Abdelnoor M, et al. Increased insulin sensitivity and fibrinolytic capacity after dietary intervention in obese women with polycystic ovary syndrome. Metabolism 1995; 44: 611-616.
- 142. Clark AM, Ledger W, Galletly C, et al. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod 1995; 10: 2705-2712.
- 143. Crave JC, Fimbel S, Lejeune H, et al. Effects of diet and metformin administration on sex hormone-binding globulin, androgens, and insulin in hirsute and obese women. J Clin Endocrinol Metab 1995; 80: 2057-2062.
- 144. Crosignani PG, Colombo M, Vegetti W, et al. Overweight and obese anovulatory patients with polycystic ovaries: parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum Reprod 2003; 18: 1928-1932.
- 145. Gambineri A, Pelusi C, Genghini S, et al. Effect of flutamide and metformin administered alone or in combination in dieting obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004; 60: 241-249.
- 146. Guzick DS, Wing R, Smith D, et al. Endocrine consequences of weight loss in obese, hyperandrogenic, anovulatory women. Fertil Steril 1994; 61: 598-604.
- 147. Holte J, Bergh T, Berne C, et al. Restored insulin sensitivity but persistently increased early insulin secretion after weight loss in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 1995; 80: 2586-2593.
- 148. Jakubowicz DJ, Nestler JE. 17 alpha-hydroxyprogesterone responses to leuprolide and serum androgens in obese women with and without polycystic ovary syndrome offer dietary weight loss. J Clin Endocrinol Metab 1997; 82: 556-560.
- 149. Kiddy DS, Hamilton-Fairley D, Bush A, et al. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 1992; 36: 105-111.
- 150. Kiddy DS, Hamilton-Fairley D, Seppälä M, et al. Diet-induced changes in sex hormone binding globulin and free testosterone in women with normal or polycystic ovaries: correlation with serum insulin and insulin-like growth factor-I. Clin Endocrinol (Oxf) 1989; 31: 757-763.
- 151. Moran LJ, Noakes M, Clifton PM, et al. C-reactive protein before and after weight loss in overweight women with and without polycystic ovary syndrome. J Clin Endocrinol Metab 2007; 92: 2944-2951.
- 152. Moran LJ, Noakes M, Clifton PM, et al. Short-term meal replacements followed by dietary macronutrient restriction enhance weight loss in polycystic ovary syndrome. Am J Clin Nutr 2006; 84: 77-87.
- 153. Pasquali R, Antenucci D, Casimirri F, et al. Clinical and hormonal characteristics of obese amenorrheic hyperandrogenic women before and after weight loss. J Clin Endocrinol Metab 1989; 68: 173-179.
- 154. Pasquali R, Gambineri A, Biscotti D, et al. Effect of long-term treatment with metformin added to hypocaloric diet on body composition, fat distribution, and androgen and insulin levels in abdominally obese women with and without the polycystic ovary syndrome. J Clin Endocrinol Metab 2000; 85: 2767-2774.
- 155. Qublan HS, Yannakoula EK, Al-Qudah MA, El-Uri FI. Dietary intervention versus metformin to improve the reproductive outcome in women with polycystic ovary syndrome. A prospective comparative study. Saudi Med J 2007; 28: 1694-1699.
- 156. Stamets K, Taylor DS, Kunselman A, et al. A randomized trial of the effects of two types of short-term hypocaloric diets on weight loss in women with polycystic ovary syndrome. Fertil Steril 2004; 81: 630-637.
- 157. Tang T, Glanville J, Hayden CJ, et al. Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo-controlled, double-blind multicentre study. Hum Reprod 2006; 21: 80-89.
- 158. Van Dam EW, Roelfsema F, Veldhuis JD, et al. Increase in daily LH secretion in response to short-term calorie restriction in obese women with PCOS. Am J Physiol Endocrinol Metab 2002; 282: E865-E872.
- 159. Wahrenberg H, Ek I, Reynisdottir S, et al. Divergent effects of weight reduction and oral anticonception treatment on adrenergic lipolysis regulation in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab 1999; 84: 2182-2187.
- 160. Hutchison SK, Stepto NK, Harrison CL, et al. Effects of exercise on insulin resistance and body composition in overweight and obese women with and without polycystic ovary syndrome. J Clin Endocrinol Metab 2011; 96: E48-E56.
- 161. Poehlman ET, Dvorak RV, DeNino WF, et al. Effects of resistance training and endurance training on insulin sensitivity in nonobese, young women: a controlled randomized trial. J Clin Endocrinol Metab 2000; 85: 2463-2468.
- 162. Ross R, Dagnone D, Jones P, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. Ann Intern Med 2000; 133: 92-103.
- 163. Moran LJ, Hutchison SK, Norman RJ, Teede HJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2011; (2): CD007506.
- 164. Palomba S, Giallauria F, Falbo A, et al. Structured exercise training programme versus hypocaloric hyperproteic diet in obese polycystic ovary syndrome patients with anovulatory infertility: a 24-week pilot study. Hum Reprod 2008; 23: 642-650.
- 165. Hession M, Rolland C, Kulkarni U, et al. Systematic review of randomized controlled trials of low-carbohydrate vs. low-fat/low-calorie diets in the management of obesity and its comorbidities. Obes Rev 2009; 10: 36-50.
- 166. Pirozzo S, Summerbell C, Cameron C, Glaziou P. Advice on low-fat diets for obesity. Cochrane Database Syst Rev 2002; (2): CD003640.
- 167. Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009; 360: 859-873.
- 168. Galletly C, Moran L, Noakes M, et al. Psychological benefits of a high-protein, low-carbohydrate diet in obese women with polycystic ovary syndrome — a pilot study. Appetite 2007; 49: 590-593.
- 169. Douglas CC, Gower BA, Darnell BE, et al. Role of diet in the treatment of polycystic ovary syndrome. Fertil Steril 2006; 85: 679-688.
- 170. Marsh KA, Steinbeck KS, Atkinson FS, et al. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr 2010; 92: 83-92.
- 171. Children’s Health Development Foundation, South Australia; Deakin University. The Australian guide to healthy eating. Canberra: Australian Government Department of Health and Ageing, 1998.
- 172. Miller W, Rollnick S. Motivational interviewing: preparing people for change. 2nd ed. New York: Guilford Press, 2002.
- 173. Avenell A, Broom J, Brown TJ, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2004; 8: iii-iv, 1-182.
- 174. Ogilvie D, Foster CE, Rothnie H, et al. Interventions to promote walking: systematic review. BMJ 2007; 334: 1204.
- 175. Dombrowski SU, Sniehotta FF, Avenell A, et al. Identifying active ingredients in complex behavioural interventions for obese adults with obesity-related co-morbidities or additional risk factors for co-morbidities: a systematic review. Health Psychol Rev 2010. [Epub ahead of print]. doi: 10.1080/17437199.2010. 513298
- 176. Gillies CL, Abrams KR, Lambert PC, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 2007; 334: 299.
- 177. Thompson RL, Summerbell CD, Hooper L, et al. Dietary advice given by a dietitian versus other health professional or self-help resources to reduce blood cholesterol. Cochrane Database Syst Rev 2003; (3): CD001366.
- 178. Shaw K, O’Rourke P, Del Mar C, Kenardy J. Psychological interventions for overweight or obesity. Cochrane Database Syst Rev 2005; (2): CD003818.
- 179. Kasim-Karakas S, Almario R, Cunningham W. Effects of protein versus simple sugar intake on weight loss in polycystic ovary syndrome (according to the National Institutes of Health criteria). Fertil Steril 2009; 92: 262-270.
- 180. Atiomo W, Read A, Golding M, et al. Local recruitment experience in a study comparing the effectiveness of a low glycaemic index diet with a low calorie healthy eating approach at achieving weight loss and reducing the risk of endometrial cancer in women with polycystic ovary syndrome (PCOS). Contemp Clin Trials 2009; 30: 451-456.
- 181. Pate RR, Pratt M, Blair SN, et al. Physical activity and public health: a recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 1995; 273: 402-407.
- 182. Richter EA, Mikines KJ, Galbo H, Kiens B. Effect of exercise on insulin action in human skeletal muscle. J Appl Physiol 1989; 66: 876-885.
- 183. Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343-1350.
- 184. Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med 1998; 49: 235-261.
- 185. Shephard RJ, Balady GJ. Exercise as cardiovascular therapy. Circulation 1999; 99: 963-972.
- 186. Cuff DJ, Meneilly GS, Martin A, et al. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care 2003; 26: 2977-2982.
- 187. Maiorana A, O’Driscoll G, Goodman C, et al. Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diabetes Res Clin Pract 2002; 56: 115-123.
- 188. Park SK, Park JH, Kwon YC, et al. The effect of combined aerobic and resistance exercise training on abdominal fat in obese middle-aged women. J Physiol Anthropol Appl Human Sci 2003; 22: 129-135.
- 189. Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med 2007; 147: 357-369.
- 190. Ross R, Bradshaw AJ. The future of obesity reduction: beyond weight loss. Nat Rev Endocrinol 2009; 5: 319-325.
- 191. Orio F, Giallauria F, Palomba S, et al. Metabolic and cardiopulmonary effects of detraining after a structured exercise training programme in young PCOS women. Clin Endocrinol (Oxf) 2008; 68: 976-981.
- 192. Thomson RL, Buckley JD, Noakes M, et al. The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 2008; 93: 3373-3380.
- 193. Brown AJ, Setji TL, Sanders LL, et al. Effects of exercise on lipoprotein particles in women with polycystic ovary syndrome. Med Sci SPorts Exerc 2009; 41: 497-504.
- 194. Bruner B, Chad K, Chizen D. Effects of exercise and nutritional counseling in women with polycystic ovary syndrome. Appl Physiol Nutr Metab 2006; 31: 384-391.
- 195. Giallauria F, Palomba S, Maresca L, et al. Exercise training improves autonomic function and inflammatory pattern in women with polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf) 2008; 69: 792-798.
- 196. Stener-Victorin E, Jedel E, Janson PO, Sverrisdottir YB. Low-frequency electroacupuncture and physical exercise decrease high muscle sympathetic nerve activity in polycystic ovary syndrome. Am J Physiol Regul Integr Comp Physiol 2009; 297: R387-R395.
- 197. Vigorito C, Giallauria F, Palomba S, et al. Beneficial effects of a three-month structured exercise training program on the cardiopulmonary functional capacity in young women with polycystic ovary syndrome. J Clin Endocrinol Metab 2007; 92: 1379-1384.
- 198. American College of Sports Medicine; American Heart Association. Physical activity and public health guidelines. Indianapolis: ACSM, 2007. http://www.acsm.org/AM/Template.cfm?Section=Home_Page&TEMPLATE=/CM/HTMLDisplay.cfm&CONTENTID=7764 (accessed Aug 2011).
- 199. Briffa T, Maiorana A, Allan R, et al; Executive Working Group and National Forum Participants. National Heart Foundation of Australia physical activity recommendations for people with cardiovascular disease. Sydney: National Heart Foundation of Australia, 2006.
- 200. Australian Government Department of Health and Ageing. National physical activity guidelines. Canberra: DoHA, 2010. http://www.health.gov.au/internet/main/publishing.nsf/Content/health-pubhlth-strateg-phys-act-guidelines (accessed Aug 2011).
- 201. Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007; 116: 1081-1093.
- 202. Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 2007; 116: 1094-1105.
- 203. Norton K, Norton L, Sadgrove D. Position statement on physical activity and exercise intensity terminology. J Sci Med Sport 2010; 13: 496-502.
- 204. Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee report 2008. Washington, DC: Department of Health and Human Services, 2008.
- 205. World Health Organization. Global strategy on diet, physical activity and health. Geneva: WHO, 2004.
- 206. World Health Organization. A guide for population-based approaches to increasing levels of physical activity: implementation of the WHO global strategy on diet, physical activity and health. Geneva: WHO, 2007.
- 207. Harrison CL, Lombard CB, Moran LJ, Teede HJ. Exercise therapy in polycystic ovary syndrome: a systematic review. Hum Reprod Update 2011; 17: 171-183.
- 208. Tang T, Lord JMM, Norman R, et al. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev 2010; (1): CD003053.
- 209. Hoeger K, Davidson K, Kochman L, et al. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab 2008; 93: 4299-4306.
- 210. Palomba S, Falbo A, Giallauria F, et al. Six weeks of structured exercise training and hypocaloric diet increases the probability of ovulation after clomiphene citrate in overweight and obese patients with polycystic ovary syndrome: a randomized controlled trial. Hum Reprod 2010; 25: 2783-2791.
- 211. Gambineri A, Patton L, Vaccina A, et al. Treatment with flutamide, metformin, and their combination added to a hypocaloric diet in overweight-obese women with polycystic ovary syndrome: a randomized, 12-month, placebo-controlled study. J Clin Endocrinol Metab 2006; 91: 3970-3980.
- 212. Hoeger KM, Kochman L, Wixom N, et al. A randomized, 48-week, placebo-controlled trial of intensive lifestyle modification and/or metformin therapy in overweight women with polycystic ovary syndrome: a pilot study. Fertil Steril 2004; 82: 421-429.
- 213. Karimzadeh MA, Javedani M. An assessment of lifestyle modification versus medical treatment with clomiphene citrate, metformin, and clomiphene citrate-metformin in patients with polycystic ovary syndrome. Fertil Steril 2010; 94: 216-220.
- 214. Otta CF, Wior M, Iraci GS, et al. Clinical, metabolic, and endocrine parameters in response to metformin and lifestyle intervention in women with polycystic ovary syndrome: a randomized, double-blind, and placebo control trial. Gynecol Endocrinol 2010; 26: 173-178.
- 215. Royal Australian and New Zealand College of Obstetricians and Gynaecologists. C-Gyn 2: ovarian stimulation in infertility. Melbourne: RANZCOG, 2008.
- 216. Shelly W, Draper MW, Krishnan V, et al. Selective estrogen receptor modulators: an update on recent clinical findings. Obstet Gynecol Surv 2008; 63: 163-181.
- 217. Adashi EY. Clomiphene citrate: mechanism(s) and site(s) of action — a hypothesis revisited. Fertil Steril 1984; 42: 331-344.
- 218. Palomba S, Falbo A, Zullo F. Management strategies for ovulation induction in women with polycystic ovary syndrome and known clomifene citrate resistance. Curr Opin Obstet Gynecol 2009; 21: 465-473.
- 219. Kafy S, Tulandi T. New advances in ovulation induction. Curr Opin Obstet Gynecol 2007; 19: 248-252.
- 220. Rossing MA, Daling JR, Weiss NS, et al. Ovarian tumours in a cohort of infertile women. N Engl J Med 1994; 331: 771-776.
- 221. Brown J, Farquhar C, Beck J, et al. Clomiphene and anti-oestrogens for ovulation induction in PCOS. Cochrane Database Syst Rev 2009; (4): CD002249.
- 222. Palomba S, Falbo A, Zullo F, Orio F Jr. Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev 2009; 30: 1-50.
- 223. Costello MF, Eden JA. A systematic review of the reproductive system effects of metformin in patients with polycystic ovary syndrome. Fertil Steril 2003; 79: 1-13.
- 224. Creanga AA, Bradley HM, McCormick C, Witkop CT. Use of metformin in polycystic ovary syndrome: a meta-analysis. Obstet Gynecol 2008; 111: 959-968.
- 225. Moll E, van der Veen F, van Wely M. The role of metformin in polycystic ovary syndrome: a systematic review. Hum Reprod Update 2007; 13: 527-537.
- 226. Johnson NP, Stewart AW, Falkiner J, et al. PCOSMIC: a multi-centre randomized trial in women with polycystic ovary syndrome evaluating metformin for infertility with clomiphene. Hum Reprod 2010; 25: 1675-1683.
- 227. Kazerooni T, Ghaffarpasand F, Kazerooni Y, et al. Short-term metformin treatment for clomiphene citrate-resistant women with polycystic ovary syndrome. Int J Gynaecol Obstet 2009; 107: 50-53.
- 228. Siebert TI, Kruger TF, Lombard C. Evaluating the equivalence of clomiphene citrate with and without metformin in ovulation induction in PCOS patients. J Assist Reprod Genet 2009; 26: 165-171.
- 229. Ben Ayed B, Dammak dit Mlik S, Ben Arab H, et al. Metformin effects on clomifene-induced ovulation in the polycystic ovary syndrome. Tunis Med 2009; 87: 43-49.
- 230. Palomba S, Pasquali R, Orio F Jr, Nestler JE. Clomiphene citrate, metformin or both as first-step approach in treating anovulatory infertility in patients with polycystic ovary syndrome (PCOS): a systematic review of head-to-head randomized controlled studies and meta-analysis. Clin Endocrinol (Oxf) 2009; 70: 311-321.
- 231. Nugent D, Vanderkerchove P, Hughes E, et al. Gonadotrophin therapy for ovulation induction in subfertility associated with polycystic ovary syndrome. Cochrane Database Syst Rev 2000; (3): CD000410.
- 232. Messinis IE. Ovulation induction: a mini review. Hum Reprod 2005; 20: 2688-2697.
- 233. Macklon N, Fauser B. The step-down protocol. In: Tarlatzis B, editor. Ovulation induction [European practice in obstetrics and gynaecology series]. Paris: Elsevier, 2002.
- 234. López E, Gunby J, Daya S, et al. Ovulation induction in women with polycystic ovary syndrome: randomized trial of clomiphene citrate versus low-dose recombinant FSH as first line therapy. Reprod Biomed Online 2004; 9: 382-390.
- 235. Homburg R, Hendriks M, Konig T, et al. Clomifene or low dose FSH for the first line treatment of anovulatory PCOS: a prospective randomised multinational study (COFFI) [oral abstract O-058]. Conference of the European Society of Human Reproduction and Embryology; 2009; Amsterdam.
- 236. Mitwally MF, Casper RF. Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril 2001; 75: 305-309.
- 237. Holzer H, Casper R, Tulandi T. A new era in ovulation induction. Fertil Steril 2006; 85: 277-284.
- 238. Healey S, Tan SL, Tulandi T, Biljan MM. Effects of letrozole on superovulation with gonadotrophins in women undergoing intrauterine insemination. Fertil Steril 2003; 80: 1325-1329.
- 239. Casper RF. Letrozole: ovulation or superovulation? Fertil Steril 2003; 80: 1335-1337.
- 240. Biljan M, Hemmings R, Brassard N. The outcome of 150 babies following the treatment with letrozole or letrozole and gonadotropins [abstract 1033]. Fertil Steril 2005; 84: O-231.
- 241. Forman R, Gill S, Moretti M, et al. Fetal safety of letrozole and clomiphene citrate for ovulation induction. J Obstet Gynaecol Can 2007; 29: 668-671.
- 242. Tulandi T, Martin J, Al-Fadhli R, et al. Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate. Fertil Steril 2006; 85: 1761-1765.
- 243. Kamath MS, Aleyamma TK, Chandy A, George K. Aromatase inhibitors in women with clomiphene citrate resistance: a randomized, double-blind, placebo-controlled trial. Fertil Steril 2010; 94: 2857-2859.
- 244. Al-Omari WR, Sulaiman WR, Al-Hadithi N. Comparison of two aromatase inhibitors in women with clomiphene-resistant polycystic ovary syndrome. Int J Gynaecol Obstet 2004; 85: 289-291.
- 245. Badawy A, Mosbah A, Shady M, et al. Anastrozole or letrozole for ovulation induction in clomiphene-resistant women with polycystic ovarian syndrome: a prospective randomized trial. Fertil Steril 2008; 89: 1209-1212.
- 246. Badawy A, Mosbah A, Tharwat A, et al. Extended letrozole therapy for ovulation induction in clomiphene-resistant women with polycystic ovary syndrome: a novel protocol. Fertil Steril 2009; 92: 236-239.
- 247. Bayar U, Kiran S, Coskun A, et al. Use of an aromatase inhibitor in patients with polycystic ovary syndrome: a prospective randomized trial. Fertil Steril 2006; 86: 1447-1451.
- 248. Badawy A, Abdel Aal I, Abulatta M, et al. Clomiphene citrate or letrozole for ovulation induction in women with polycystic ovarian syndrome: a prospective randomized trial. Fertil Steril 2009; 92: 849-852.
- 249. Dehbashi S, Kazerooni T, Robati M, et al. Comparison of the effects of letrozole and clomiphene citrate on ovulation and pregnancy rate in patients with polycystic ovary syndrome. Iran J Med Sci 2009; 34: 23-28.
- 250. Begum MR, Ferdous J, Begum A, Quadir E. Comparison of efficacy of aromatase inhibitor and clomiphene citrate in induction of ovulation in polycystic ovarian syndrome. Fertil Steril 2009; 92: 853-857.
- 251. Atay V, Cam C, Muhcu M, et al. Comparison of letrozole and clomiphene citrate in women with polycystic ovaries undergoing ovarian stimulation. J Int Med Res 2006; 34: 73-76.
- 252. Zeinalzadeh M, Basirat Z, Esmailpour M. Efficacy of letrozole in ovulation induction compared to that of clomiphene citrate in patients with polycystic ovarian syndrome. J Reprod Med 2010; 55: 36-40.
- 253. Abu Hashim H, Shokeir T, Badawy A. Letrozole versus combined metformin and clomiphene citrate for ovulation induction in clomiphene-resistant women with polycystic ovary syndrome: a randomized controlled trial. Fertil Steril 2010; 94: 1405-1409.
- 254. Gjönnaess H. Polycystic ovarian syndrome treated by ovarian electrocautery through the laparoscope. Fertil Steril 1984; 41: 20-25.
- 255. Farquhar C, Lilford R, Marjoribanks J, Van dekerckhove P. Laparoscopic “drilling” by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev 2007; (3): CD001122.
- 256. Palomba S, Falbo A, Battista L, et al. Laparoscopic ovarian diathermy vs clomiphene citrate plus metformin as second-line strategy for infertile anovulatory patients with polycystic ovary syndrome: a randomized controlled trial. Am J Obstet Gynecol 2010; 202: e1-e8.
- 257. Hamed HO, Hasan AF, Ahmed OG, Ahmed MA. Metformin versus laparoscopic ovarian drilling in clomiphene- and insulin-resistant women with polycystic ovary syndrome. Int J Gynaecol Obstet 2010; 108: 143-147.
- 258. Palomba S, Orio F Jr, Nardo LG, et al. Metformin administration versus laparoscopic ovarian diathermy in clomiphene citrate-resistant women with polycystic ovary syndrome: a prospective parallel randomized double-blind placebo-controlled trial. J Clin Endocrinol Metab 2004; 89: 4801-4809.
- 259. Palomba S, Orio F Jr, Falbo A, et al. Plasminogen activator inhibitor 1 and miscarriage after metformin treatment and laparoscopic ovarian drilling in patients with polycystic ovary syndrome. Fertil Steril 2005; 84: 761-765.
- 260. Amer SA, Li TC, Metwally M, et al. Randomized controlled trial comparing laparoscopic ovarian diathermy with clomiphene citrate as a first-line method of ovulation induction in women with polycystic ovary syndrome. Hum Reprod 2009; 24: 219-225.
- 261. Farquhar C, Williamson K, Gudex G, et al. A randomized controlled trial of laparoscopic ovarian diathermy versus gonadotrophin therapy for women with clomiphene citrate-resistant polycystic ovary syndrome. Fertil Steril 2002; 78: 404-411.
- 262. Apovian CM, Cummings S, Anderson W, et al. Best practice updates for multidisciplinary care in weight loss surgery. Obesity (Silver Spring) 2009; 17: 871-879.
- 263. Heber D, Greenway FL, Kaplan LM, et al. Endocrine and nutritional management of the post-bariatric surgery patient: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2010; 95: 4823-4843.
- 264. Shah DK, Ginsburg ES. Bariatric surgery and fertility. Curr Opin Obstet Gynecol 2010; 22: 248-254.
- 265. American College of Obstetrics and Gynecology. Clinical management guidelines for obstetrician-gynecologists. Washington, DC: ACOG, 2009.
- 266. Picot J, Jones J, Colquitt J, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 2009; 13: 1-190, 215-357, iii-iv.
- 267. Scottish Intercollegiate Guidelines Network. Management of obesity: a national clinical guideline. Edinburgh: SIGN, 2010.
- 268. National Institute for Health and Clinical Excellence. NICE clinical guideline 43. Obesity: guidance on the prevention, identification, assessment and management of overweight and obesity in adults and children. London, 2006.
- 269. Nilsen RM, Vollset SE, Gjessing HK, et al. Patterns and predictors of folic acid supplement use among pregnant women: the Norwegian Mother and Child Cohort Study. Am J Clin Nutr 2006; 84: 1134-1141.
- 270. Mechanick JI, Kushner RF, Sugerman HJ, et al; American Association of Clinical Endocrinologists; Obesity Society; American Society for Metabolic & Bariatric Surgery. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Obesity (Silver Spring) 2009; 17 Suppl 1: S1-S70, v.
- 271. Rubino F, Kaplan LM, Schauer PR, Cummings DE. The Diabetes Surgery Summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg 2010; 251: 399-405.





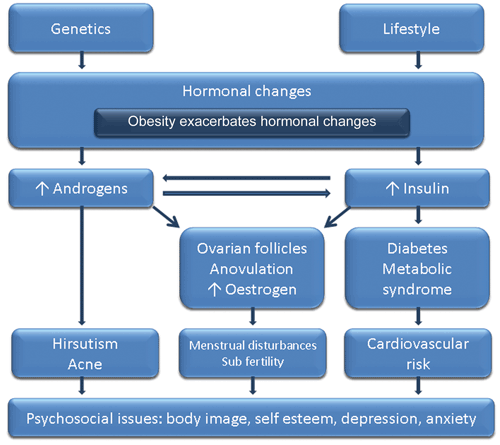
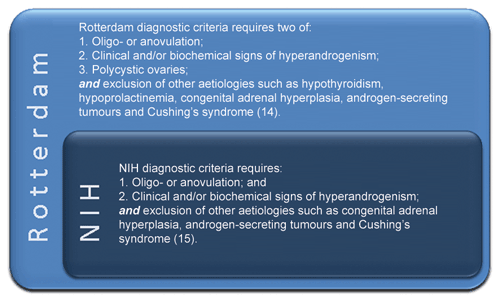
Rob Norman has part-ownership in Fertility SA, a company providing fertility and IVF services in Adelaide; has received speaker fees from MSD Australia and Merck Serono Australia, received honoraria from MSD–Schering-Plough and Merck Serono Australia; and is a member of the MSD–Schering-Plough Advisory Board. Bronwyn Stuckey has received travel funding from Bayer Australia and Schering- Plough, both of which have interests in the oral contraceptives Diane-35 and Yasmin, which are commonly prescribed to women with PCOS. Michael Costello has shares in IVF Australia; was the recipient of a grant from Schering-Plough in 2002 for research project unrelated to PCOS (Schering-Plough manufactures FSH injections for ovulation induction and ovarian stimulation); received sponsorship to attend and present at national and international scientific meetings on a broad range of topics determined by the individual conference organising committees, from pharmaceutical companies (Merck Serono Australia and Schering-Plough) who have a commercial interest in PCOS treatment products or guidelines; and is a member of the MSD–Schering-Plough Advisory Board.