A husband and wife became unwell after eating a fish from the Calder River in northern Western Australia. Gnathostomiasis was diagnosed, and treated with ivermectin and albendazole. Serological testing was positive for gnathostomiasis, and there has been no recurrence. These appear to be the first proven endemically acquired cases of gnathostomiasis in Australia, and demonstrate the difficulties in diagnosis and treatment. (MJA 2011; 195: 42-44)
A 52-year-old man developed epigastric discomfort, nausea, diarrhoea and lethargy 10 days after eating a fish (identified by the patient as “black bream”, possibly Acanthopagrus berda or Hephaestus jenkinsi) caught from the Calder River in northern Western Australia (Box 1). The fish had been pan-fried whole over a camp fire, but the duration and thoroughness of cooking is unclear. The patient’s epigastric discomfort persisted, followed a week later by fevers and myalgia and then pruritic subcutaneous swellings and skin thickening over his chest and abdomen. He had no response to a prescribed course of antibiotics. His abdominal symptoms and myalgia continued and, over the next 2 months, multiple episodic swellings developed over his abdomen. The swellings progressed to involve both thighs and were associated with feelings of movement under his skin.
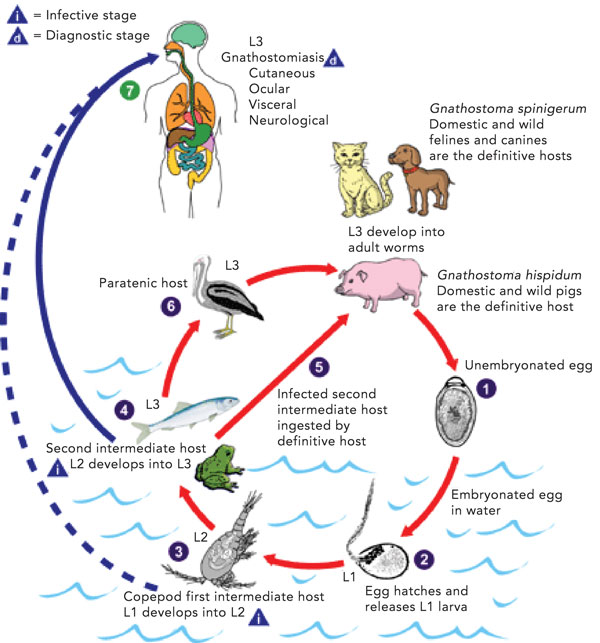
Gnathostomiasis is a foodborne zoonosis, a clinical syndrome caused most commonly by infection with the larvae of Gnathostoma spinigerum, but also by several other Gnathostoma species.1,2 Humans may become accidental hosts after ingesting third-stage larvae (Box 2). The larvae are unable to mature further in humans, and they migrate through visceral and cutaneous tissues. Larvae may be found in a range of intermediate and paratenic hosts including freshwater fish, snakes, frogs, snails and fowl.1,3 Consequently, the disease is endemic where these foods are consumed raw or undercooked, including South-East Asia and Japan, but more recently recognised in Latin America, China, India, Africa and in travellers returning from these areas.1,2 Locally acquired infection in Australia has not previously been confirmed.4 The Gnathostoma life cycle is illustrated in Box 2.
Visceral disease may involve almost any part of the body. Pulmonary disease may manifest as a cough, pleuritic chest pain, haemoptysis, lobar consolidation or pleural effusions. Gastrointestinal disease may be asymptomatic, but is more frequently associated with sharp abdominal pains or inflammatory masses, or may mimic an “acute surgical abdomen”. Ocular disease has a variety of manifestations and often allows direct visualisation of the larvae. Untreated, gnathostomiasis of the central nervous system (CNS) is associated with the highest mortality (8%–25%), and 30% of survivors have long-term sequelae.1 Typically, symptoms begin with acute radicular pain or headache, lasting up to 5 days, as the larva penetrates the CNS via spinal cord nerve roots. Focal paralysis and cranial nerve palsies usually follow, and may progress to eosinophilic meningoencephalitis or encephalomyelitis. Subarachnoid haemorrhage and other vascular complications are less common presentations of CNS gnathostomiasis. Imaging reveals the haemorrhagic migratory tracts of the larvae. The main differential diagnosis for CNS disease is Angiostrongylus cantonensis.
Definitive diagnosis of gnathostomiasis requires parasite extraction and identification, however small parasite size (2–3 mm) makes this impractical, and thus is no longer recommended. Therefore, gnathostomiasis is a clinical diagnosis, supported by epidemiological history, blood eosinophilia (although alone not sensitive or specific) and serological testing. Serological tests have surpassed non-specific antigen injection techniques, and include immunoblot testing (which detects antibodies to specific L3 antigen with a molecular mass of 24 kDa) and ELISA (which detects IgG1 or IgG2 to the crude L3 antigen).6-8
The immunoblot test appears to be the most specific, but is difficult to perform.6-8 In a series of four patients with parasitologically confirmed gnathostomiasis, and 15 patients with a presumptive diagnosis of gnathostomiasis, the 24 kDa L3 antigen immunoblot test had a sensitivity of 100% for parasitologically confirmed gnathostomiasis and 33% for presumed gnathostomiasis.8 The authors commented that the lower sensitivity in presumptively diagnosed cases was probably due to initial incorrect diagnoses. In the same series, 64 patients with other parasitic infections and 19 healthy control subjects were also tested, with only one positive result, giving a specificity of 99%.8
An ELISA to detect subclass immunoglobulin levels for crude L3 antigen, avoiding the difficult purification step, has been proposed as an alternative diagnostic test. Detection of IgG1 has the greatest sensitivity (98%) making it an attractive initial test, and IgG2 does not appear to cross-react with other parasitic species, thus providing the best specificity (88%) for diagnostic confirmation of gnathostomiasis.7 Neither test is available commercially, but both the immunoblot and ELISA are performed at the Department of Helminthology, Mahidol University, Bangkok, Thailand. Nevertheless, serological investigations for gnathostomiasis have limited validation, making precise estimates of sensitivity and specificity difficult. Cross-reactions may occur, so caution with interpretation is paramount. In the patients described, an evaluation for other parasitic infections was made in order to reduce the possibility of a false positive result.
Data comparing the outcomes of treatment regimens are limited to observational studies only. In a series of 49 patients treated with albendazole 400 mg twice daily for 21 days, over 93% achieved a cure at 6 months.9 In the same series, the efficacy was similar in 21 patients treated with ivermectin 0.2 mg/kg as a single dose.9 There are few data comparing combinations of ivermectin and albendazole. Longer observational studies indicate that treatment failures are common, as demonstrated in the two patients above, and thus repeat treatment is recommended if symptoms recur.10
Proven endemic human gnathostomiasis has not previously been reported in Australia, although cases suspected to have been locally acquired were described in the 1970s (non-specific antigen injection was used for confirmation).4 Gnathostoma infection of mammals has been described in Australia.11,12 Therefore, we believe these cases are the first confirmed cases of locally acquired human gnathostomiasis in Australia. The two patients had clinical syndromes compatible with gnathostomiasis, associated blood eosinophilia, positive serological results and supportive epidemiological history, and they responded to treatment. Importantly, neither of the patients had previously travelled outside Australia. Weaknesses of this report include that we were unable to definitively identify the fish species or determine how thoroughly the fish was cooked.
Provenance: Not commissioned; externally peer reviewed.
- Cameron J Jeremiah1
- Chanad S Harangozo2
- Andrew J Fuller1
- 1 The Alfred Hospital, Melbourne, VIC.
- 2 South Eastern Private Hospital, Melbourne, VIC.
- 1. Herman JS, Chiodini PL. Gnathostomiasis: another emerging imported disease. Clin Microbiol Rev 2009; 22: 484-492.
- 2. Nawa Y, Hatz C, Blum J. Sushi delights and parasites: the risk of fishborne and foodborne parasitic zoonoses in Asia. Clin Infect Dis 2005; 41: 1297-1303.
- 3. Ramirez-Avila L, Slome S, Schuster FL, et al. Eosinophilic meningitis due to Angiostrongylus and Gnathostoma species. Clin Infect Dis 2009; 48: 322-327.
- 4. Moorhouse DE, Bhaibulaya M, Jones HI. Suspected human gnathostomiasis in Queensland. Med J Aust 1970; 2: 250.
- 5. DPDx, Laboratory Identification of Parasites of Public Health Concern. Adapted from Gnathostomiasis: life cycle. Centers for Disease Control and Prevention. http://www.dpd.cdc.gov/dpdx/HTML/gnathostomiasis.htm (accessed Feb 2011).
- 6. Nopparatana C, Setasuban P, Chaicumpa W, Tapchaisri P. Purification of Gnathostoma spinigerum specific antigen and immunodiagnosis of human gnathostomiasis. Int J Parasitol 1991; 21: 677-687.
- 7. Nuchprayoon S, Sanprasert V, Suntravat M, et al. Study of specific IgG subclass antibodies for diagnosis of Gnathostoma spinigerum. Parasitol Res 2003; 91: 137-143.
- 8. Tapchaisri P, Nopparatana C, Chaicumpa W, Setasuban P. Specific antigen of Gnathostoma spinigerum for immunodiagnosis of human gnathostomiasis. Int J Parasitol 1991; 21: 315-319.
- 9. Nontasut P, Bussaratid V, Chullawichit S, et al. Comparison of ivermectin and albendazole treatment for gnathostomiasis. Southeast Asian J Trop Med Public Health 2000; 31: 374-377.
- 10. Strady C, Dekumyoy P, Clement-Rigolet M, et al. Long-term follow-up of imported gnathostomiasis shows frequent treatment failure. Am J Trop Med Hyg 2009; 80: 33-35.
- 11. Smales LR. Species of Gnathostoma (Nematoda: Spiruroidea) from bandicoots and dasyurids (Marsupialia) from Australia [brief communication]. Transact Roy Soc South Aust 1999; 123: 83-84.
- 12. Adams PJ. Parasites of feral cats and native fauna from Western Australia: the application of molecular techniques for the study of parasitic infections in Australian wildlife [doctoral thesis]. Perth: Murdoch University, 2003. http://researchrepository.murdoch.edu.au/29/ (accessed Jan 2011).