Around two billion individuals worldwide have latent tuberculosis infection (LTBI)1 — that is, asymptomatic infection with Mycobacterium tuberculosis. Diagnosis of this condition is an important intervention in tuberculosis control, as treatment markedly reduces the potential for dormant infection to progress to active disease.2 Isoniazid for 6 to 12 months is 60%–90% effective in preventing this and is generally well tolerated.3 However, side effects occur, leading to debate over its appropriateness and the optimal duration of therapy.4 Moreover, rates of LTBI treatment completion are low, and targeting groups who are most at risk of reactivation, such as refugees, is recommended.5
The Centre for Disease Control, NT (CDC-NT) screens for LTBI in all refugees, except those with documented past tuberculosis, at the time of their arrival in Darwin. Screening is by intradermal injection of tuberculin purified protein derivative 0.1 mL (tuberculin skin test [TST]). In refugees who are immunocompetent, LTBI is defined as ≥ 10 mm induration; BCG vaccination status is not used to adjust the diagnostic threshold in this high-risk group. Those with positive results are assessed for active tuberculosis by chest x-ray and medical review. Our standard treatment for LTBI is 9 months of daily isoniazid (10–15 mg/kg in children [≤ 11 years] and 5 mg/kg in adults, to a maximum of 300 mg for both) with pyridoxine (< 5 years, 6.25 mg; 5–11 years, 12.5 mg; > 11 years, 25 mg).6 Patients receive monthly nursing review throughout treatment, with self-reported treatment compliance recorded at each visit. Monthly serum liver enzyme levels are measured in patients aged ≥ 35 years and those with risk factors for liver disease.
Outcomes and clinical management were compared with the recommendations and protocols of the jurisdiction where management was provided. For most patients, care was provided by the CDC-NT and management was compared with NT guidelines.6 These patients were prescribed a 9-month isoniazid regimen. The CDC-NT’s goal of ≥ 80% of 9-month doses taken within a 12-month period was used to define treatment success. Where treatment was outside the NT (three in Western Australia, one in Queensland), the local definition of treatment completion for that state was used.
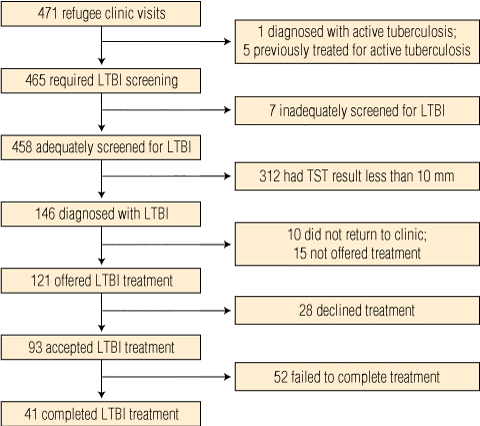
From 1 February 2006 to 31 January 2009, the CDC-NT refugee clinic saw 471 refugees, all holding Humanitarian Program visas. Their demographic characteristics and aspects of screening on arrival are shown in Box 1 and Box 2. Three of six WHO regions were represented, and Burma, the Democratic Republic of the Congo and Liberia were the most common countries of birth. Most refugees were born in the WHO region of Africa; 81.1% were born in continental Africa.
Outcomes of assessment and treatment for all individuals are shown in Box 3. One refugee required treatment for clinically active, smear-negative, culture-negative pulmonary tuberculosis. A further five gave a history of previously treated active tuberculosis, leaving 465 for TST administration. Seven of these people did not return for TST reading, leaving 458 of those eligible for testing (98.5%) adequately screened for LTBI.
Of the 458 refugees adequately assessed, 146 (31.9%) were diagnosed with LTBI, while the remaining 312 returned negative results, including 246 with readings of zero. For refugees with non-zero TST results, the median induration diameter was 12 mm (IQR, 8–16 mm). The median interval between arrival in Australia and TST result was 22 days (IQR, 14–34 days). A higher prevalence of LTBI was seen among those born in the WHO Eastern Mediterranean region, male refugees and older individuals (Box 4).
Of 146 refugees diagnosed with LTBI, 10 did not attend follow-up and 15 were deemed unsuitable for therapy by the treating clinician; three because of medical contraindications, seven because of pregnancy or breastfeeding, and five who were considered to be “low risk”. The remaining 121 were offered preventive therapy and 93 (76.9%) accepted, while 28 (23.1%) refused, or parental consent was not granted. Refugees from the Eastern Mediterranean area were least likely to accept treatment (Box 4). Daily isoniazid was prescribed for 89 refugees, while four were prescribed three times per week directly observed preventive therapy.
Of 93 refugees who accepted treatment, 41 (44.1%) met the study end point for treatment completion. Thirty-eight (40.9%) of these successfully completed a 9-month course, while three who were treated interstate met the study end point by completing 6 months of treatment. Overall, sufficient doses to complete 6 months of therapy were taken by 46 refugees (49.5%). Treatment completion was most often achieved in younger refugees and refugees from South-East Asia (Box 4).
The prevalence of active tuberculosis among refugees in this study was relatively low,7 at 2.1 per 1000 (one refugee in 471), probably reflecting effective pre-departure screening and timely assessment on arrival, and implying little immediate risk of infection to the general community. However, in the developed world, most cases of active tuberculosis result from reactivation of LTBI, and LTBI is increasingly recognised as an important reservoir for tuberculosis globally.8 In Australia, most LTBI diagnoses are made in foreign-born individuals,9 and refugees are at particularly high risk, as their exposure often occurs shortly before arrival in Australia.10 For these reasons, most developed countries target new immigrants and refugees for tuberculosis screening on entry, although approaches vary considerably.11,12
Our finding of LTBI in 31.9% of refugees is comparable to other Australian data.13,14 It is likely that the association between increasing age and LTBI reflects cumulative exposure in highly endemic regions. We were unable to assess the impact of HIV infection and BCG vaccination status, which are potentially important associations. LTBI prevalence rates in countries of birth are unknown, but regional prevalences of active tuberculosis in 2007 were: Africa, 475 cases per 100 000; Eastern Mediterranean, 139; and South-East Asia, 280.15 While we found a higher prevalence of LTBI in refugees from the Eastern Mediterranean region, most of these individuals were from Somalia and Sudan (reflecting recent political unrest), which had prevalences of active tuberculosis of 494 and 206 per 100 000, respectively, in 2009.16,17
In 13 of the 15 refugees in our study who were not offered LTBI treatment, a reasonable contraindication could be substantiated — most often age, pregnancy, breastfeeding or comorbidities. The refusal rate for LTBI treatment in our study (23.1%) is comparable to previous studies, including the large North American Tuberculosis Epidemiologic Studies Consortium (TBESC) study (17.1% overall, 23.4% of foreign-born individuals).18
The efficacy of prophylactic isoniazid increases with treatment adherence.3 The rate of failure to complete treatment in our study (55.9%) was similar to the rate in the TBESC study (52.5%), regardless of overseas-born status.18 One study in a group that included a high proportion of overseas-born individuals found an overall completion rate of 38.6%;19 while others20,21 included high proportions of immigrants but differed in their assessment of the effect of being foreign-born. A systematic review of studies in the United States and Canada concluded that individual trials were inconsistent, but overall results for treatment completion were suboptimal and few factors consistently predicted adherence.22 A study of refugees in Canada23 reported completion of 6 months of therapy in 69% (24/35), although few studies have specifically examined refugee groups.
Qualitative studies of tuberculosis treatment have demonstrated personal, structural, social and health-service factors to be important.24 However, few data are available for LTBI treatment. High rates of completion for LTBI treatment have been attained through cultural approaches, such as linking case managers to refugees of the same cultural background.25
1 Baseline characteristics of 471 refugees who underwent health screening at the CDC-NT clinic, 1 Feb 2006 – 31 Jan 2009
2 Overall results for refugees who underwent health screening at the CDC-NT clinic, 1 Feb 2006 – 31 Jan 2009
3 Summary of outcomes and numbers of newly arrived refugees included at each stage of assessment and treatment for latent tuberculosis infection (LTBI)
 |
|
|
Provenance: Not commissioned; externally peer reviewed.
Received 29 September 2010, accepted 4 April 2011
- James M Trauer1
- Vicki L Krause2
- Centre for Disease Control, Darwin, NT.
We gratefully acknowledge all CDC-NT staff involved in this ongoing project since 2006. Particular thanks go to Natalie Gray, Lynette Kerr, Julie Graham, Kerryn Coleman and Meredith Hansen-Knarhoi.
None identified.
- 1. Lönnroth K, Castro KG, Chakaya JM, et al. Tuberculosis control and elimination 2010–2050: cure, care, and social development. Lancet 2010; 375: 1814-1829.
- 2. Al-Orainey IO. Diagnosis of latent tuberculosis: can we do better? Ann Thorac Med 2009; 4: 5-9.
- 3. Smieja MJ, Marchett CA, Cook DJ, et al. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst Rev 2000; (2): CD001363.
- 4. Moulding T. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis 2000; 4: 485-487.
- 5. Horsburgh CR. Priorities for the treatment of latent tuberculosis in the United States. N Engl J Med 2004; 350: 2060-2067.
- 6. Centre for Disease Control. Guidelines for the control of tuberculosis in the Northern Territory. 4th ed. Darwin: Northern Territory Government Department of Health and Community Services, 2008.
- 7. Arshad S, Bavan L, Gajari K, et al. Active screening at entry for tuberculosis among new immigrants: a systematic review and meta-analysis. Eur Respir J 2010; 35: 1336-1345.
- 8. Vynnycky E, Borgdorff MW, Leung CC, et al. Limited impact of tuberculosis control in Hong Kong: attributable to high risks of reactivation disease. Epidemiol Infect 2008; 136: 943-952.
- 9. Victorian Government Department of Human Services. Management, control and prevention of tuberculosis: guidelines for health care providers 2002–2005. Melbourne: DoHS, 2002. http://www.health.vic.gov.au/__data/assets/pdf_file/0006/19986/tb_mgmt_guide.pdf (accessed Oct 2009).
- 10. Marks GB, Bai J, Simpson SE, et al. Incidence of tuberculosis among a cohort of tuberculin-positive refugees in Australia: reappraising the estimates of risk. Am J Respir Crit Care Med 2000; 162: 1851-1854.
- 11. Sterling TR, Bethel J, Goldberg S, et al; Tuberculosis Epidemiologic Studies Consortium. The scope and impact of treatment of latent tuberculosis infection in the United States and Canada. Am J Respir Crit Care Med 2006; 173: 927-931.
- 12. Coker R, Bell A, Pitman R, et al. Tuberculosis screening in migrants in selected European countries shows wide disparities. Eur Respir J 2006; 27: 801-807.
- 13. Tiong AC, Patel MS, Gardiner J, et al. Health issues in newly arrived African refugees attending general practice clinics in Melbourne. Med J Aust 2006; 185: 602-606.
- 14. Lucas M, Nicol P, McKinnon E, et al. A prospective large-scale study of methods for the detection of latent Mycobacterium tuberculosis infection in refugee children. Thorax 2010; 65: 442-448.
- 15. World Health Organization. Global tuberculosis control 2009: epidemiology, strategy, financing. Geneva: WHO Press, 2009.
- 16. World Health Organization. TB profile: Somalia. https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG 2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=SO&outtype=pdf (accessed May 2011).
- 17. World Health Organization. TB profile: Sudan. https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2 %2FPROD%2FEXT%2FTBCountryProfile&ISO2=SD&outtype=pdf (accessed May 2011).
- 18. Horsburgh CR Jr, Goldberg S, Bethel J, et al; Tuberculosis Epidemiologic Studies Consortium. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest 2010; 137: 401-409.
- 19. Parsyan AE, Saukkonen J, Barry MA, et al. Predictors of failure to complete treatment for latent tuberculosis infection. J Infect 2007; 54: 262-266.
- 20. LoBue PA, Moser KS. Use of isoniazid for latent tuberculosis infection in a public health clinic. Am J Respir Crit Care Med 2003; 168: 443.
- 21. Li J, Munsiff SS, Tarantino T, et al. Adherence to treatment of latent tuberculosis infection in a clinical population in New York City. Int J Infect Dis 2010; 14: e292-e297.
- 22. Hirsch-Moverman Y, Daftary A, Franks J, et al. Adherence to treatment for latent tuberculosis infection: systematic review of studies in the US and Canada. Int J Tuberc Lung Dis 2008; 12: 1235-1254.
- 23. Levesque JF, Dongier P, Brassard P, et al. Acceptance of screening and completion of treatment for latent tuberculosis infection among refugee claimants in Canada. Int J Tuberc Lung Dis 2004; 8: 711-717.
- 24. Munro SA, Lewin SA, Smith HJ, et al. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med 2007; 4: e238.
- 25. Goldberg SV, Wallace J, Jackson JC, et al. Cultural case management of latent tuberculosis infection. Int J Tuberc Lung Dis 2004; 8: 76-82.





Abstract
Objectives: To assess the prevalence of latent tuberculosis infection (LTBI) in recently arrived refugees in the Northern Territory and to obtain comprehensive data for rates of treatment acceptance and completion for this condition.
Design, setting and participants: Prospective data collection and follow-up of all 471 newly arrived refugees seen at the Centre for Disease Control, NT refugee health clinic from February 2006 to January 2009.
Main outcome measures: Rates of LTBI determined by tuberculin skin testing; subsequent assessment and treatment compared with local protocols.
Results: 458 of 465 eligible refugees were adequately assessed for LTBI, of whom 146 (31.9%) were diagnosed with LTBI. Older age, male sex and World Health Organization Eastern Mediterranean region of birth were associated with increased prevalences of LTBI. Of the refugees diagnosed with LTBI, 10 failed to attend for follow-up and 15 were not offered treatment. Isoniazid therapy was accepted by 93 of 121 refugees (76.9%), and 41 of these (44.1%) completed treatment. The most common reasons for discontinuation of therapy were medication-related side effects (most often gastrointestinal) and loss to follow-up. Increasing age was associated with failure to complete treatment.
Conclusion: Outcomes of assessment and treatment for LTBI in newly arrived refugees in the NT are comparable to those for other target groups screened in developed countries. Loss to follow-up caused significant attrition in numbers, but complete data were obtained for a large proportion of eligible refugees. Most refugees who are offered treatment for LTBI accept, but less than half complete treatment.