Acatheter inserted with the tip in a central vein is called a central venous line (CVL). Correct insertion technique is important in prevention of central line-associated bacteraemia (CLAB). Patients in intensive care units (ICUs) are at high risk of CLAB, which increases mortality and morbidity rates, as well as costs.1 Yet, CLAB can be prevented.2,3 Since 2000, several studies have shown that educational strategies for aseptic insertion of CVLs lower rates of CLAB.4-7
In 2002, the Centers for Disease Control and Prevention (CDC) published Guidelines for the prevention of intravascular catheter-related infections.8 These and similar guidelines have been successfully applied in ICUs using collaborative methods.5,9 For example, Pronovost and colleagues showed that a regimen of hand washing, full barrier precautions, use of an alcohol-based chlorhexidine skin preparation, avoidance of femoral insertion, and early removal of the CVL resulted in an 81% reduction in the mean rate of CLAB.9
All 37 ICUs in NSW public hospitals were invited to participate — 10 tertiary, 12 metropolitan, 13 rural and two paediatric units. The Intensive Care Coordination and Monitoring Unit engaged with intensivists working in ICUs. The Clinical Excellence Commission provided expertise in collaborative methods, and the project team facilitated data collection and generated reports. Project governance was provided by a steering committee, with stakeholder representation. Membership and evaluation are described in the final report of the CLAB ICU collaborative.10
A multidisciplinary expert group was convened to develop a guideline for CVL insertion.11 It was based on existing guidelines and the premise that CLAB is causally related to insertion technique — compliance with hand hygiene, skin preparation and full barrier precautions are essential. A checklist including the “patient bundle” and the “clinician bundle” (Box 1) was developed to support compliance with the guideline and was also used to collect data.12 Compliance with each element was recorded by clinicians or assistants on completion of CVL insertion. Complying with a bundle required compliance with all elements. Other data recorded included insertion site, type of CVL and any complications. The checklist was completed for CVLs inserted in patients who had been admitted to an ICU for ≥ 24 hours.
CLAB episodes were notified by means of the checklist. Only CLAB in patients in the ICU, or within 24 hours of transfer out of the ICU, was reported. The definition of CLAB used was the NSW Department of Health surveillance definition (2005)13 and the definition of the CDC,8 with the exception of the time variable in relation to ICU discharge, which was changed from 48 to 24 hours to minimise the administrative burden on ICUs (Box 2).
Personnel from the Intensive Care Coordination and Monitoring Unit and the Clinical Excellence Commission promoted the intervention to intensive care clinicians. ICUs were asked to form improvement teams with physician and nursing representatives from within existing staff. Other engagement strategies are described in the final report of the CLAB ICU collaborative.10
We received 11 575 checklists for CVLs inserted in ICUs: 10 890 checklists provided line type, 10 850 provided insertion site and 10 575 provided line type and insertion and removal dates. The CVLs included centrally inserted (72.6%; 7907) and peripherally inserted (13.5%; 1467) CVLs, dialysis catheters (11.9%; 1296), and other/not specified CVLs (2.0%; 220). There was no significant difference (P = 0.998) in choice of insertion site for CVLs in the run-in period and the final 6 months’ analysis period (Box 3).
Centrally inserted CVLs contributed 78.2% of all line-days over the intervention period. During the first 12 months, these CVLs remained in situ for the same period as in the final 6 months (Box 4). The median in-situ period for peripherally inserted CVLs extended by 2 days to 8 days in the final 6 months; however, peripherally inserted CVLs represented only 8.7% of the total line-days over the 18-month study period. Dialysis CVLs and other unspecified CVLs contributed 12.2% and 0.9%, respectively, of all line-days, and their median in-situ period decreased by 1 day during the final 6 months of the intervention period.
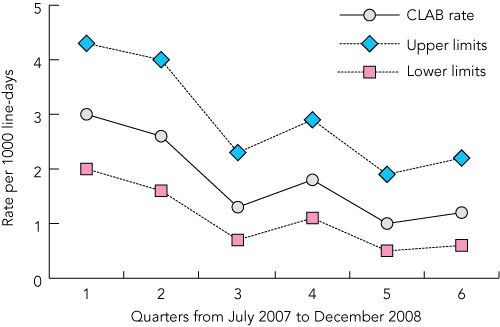
The 60% reduction in CLAB rates achieved during the intervention period — from 3.0 per 1000 line-days to 1.2 per 1000 line-days in NSW — is similar to other CLAB rate reductions published. Pronovost et al reported a reduction to a mean of 1.4 per 1000 line-days at 16–18 months.9 In 32 hospitals (69 ICUs) in the United States using similar methods, the CLAB rate was reduced by 68% to 1.36 per 1000 line-days over a 4-year period.14 Comparable results have been obtained in other US hospitals.15,16
Compliance with aseptic insertion improved significantly during the intervention, indicating that the use of bundles and checklists may improve outcomes.17 The improved compliance occurred within the broader context of a CLAB awareness campaign, and the collaborative encouraged ICUs to improve all aspects of CVL insertion and management. We believe that greater awareness among ICU clinicians of the incidence and surveillance requirements of CLAB contributed to the overall reduction in CLAB rates. The rates of earlier removal of CVLs based on in-situ time and the use of femoral insertion did not change significantly between the run-in period (first 12 months) and the analysis period (final 6 months), suggesting that neither contributed to the reduced CLAB rate in our dataset, contrary to other reports.18-21
Compliance with the clinician bundle contributed to a decreased CLAB rate. Non-compliance with the clinician bundle was attributable in 94% of cases to non-compliance with the hat–mask–eyewear element. Hat wearing was identified as the contentious component of the bundle. Clinicians cited a lack of evidence as a reason to omit hats, and four ICUs elected to omit their use as standard practice for CVL insertion. There is no direct evidence linking hat wearing to CLAB. However, hats have been routinely cited as an element of clinician preparation in reports analysing reduction in CLAB rates.22-24 Compliance with both bundles was promoted in this project, and evidence suggests that hat wearing is a required step during CVL insertion, with our data indicating that the risk of CLAB increases if hats are not worn. At best this is an association, and not wearing a hat may be a surrogate for cultural or other practice issues.
Some ICUs resisted the requirement for clinical staff to follow up CVLs and submit checklists. There is inconsistent data collection and audit in NSW ICUs,25 with limited staff allocated to quality activities in individual ICUs; in nine referral ICUs surveyed, there were only 2.2 full-time-equivalent (FTE) physicians (three ICUs), 4.73 FTE nursing staff (four ICUs), and 0.4 FTE other staff (one ICU).26 As a data-quality initiative, two tertiary ICUs and one metropolitan ICU were asked to review data capture: all three reported data capture greater than 90%. This quality test was not completed by all sites, and at least one tertiary unit had low data capture.
2 Definition of central line-associated bacteraemia (CLAB)*
AND at least one of the following:
* Based on the surveillance definition of the New South Wales Department of Health (2005),13 and the definition of the Centres for Disease Control and Prevention.8
Provenance: Not commissioned; externally peer reviewed.
Received 17 August 2010, accepted 13 January 2011
- Anthony R Burrell1,2
- Mary-Louise McLaws3
- Margherita Murgo2
- Eda Calabria2
- Annette C Pantle2
- Robert Herkes1,4
- 1 Intensive Care Coordination and Monitoring Unit, NSW Department of Health, Sydney, NSW.
- 2 Clinical Excellence Commission, Sydney, NSW.
- 3 School of Public Health and Community Medicine, University of New South Wales, Sydney, NSW.
- 4 Intensive Care Service, Royal Prince Alfred Hospital, Sydney, NSW.
The NSW Department of Health provided funding to the Clinical Excellence Commission for project management.
None identified.
- 1. Taconelli E, Smith G, Hieke K, et al. Epidemiology, medical outcomes and costs of catheter-related bloodstream infections in intensive care units of four European countries: literature- and registry-based estimates. J Hosp Infect 2009; 72: 97-103.
- 2. Ramritu P, Halton K, Cook D, et al. Catheter-related bloodstream infections in intensive care units: a systematic review with meta-analysis. J Adv Nurs 2008; 62: 3-21.
- 3. Raad I, Hohn D, Gilbreath B, et al. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol 1994; 15: 231-238.
- 4. Eggimann P. Prevention of intravascular catheter infection. Curr Opin Infect Dis 2007; 20: 360-369.
- 5. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med 2004; 32: 2014-2020.
- 6. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest 2004; 126: 1612-1618.
- 7. Sheretz R, Ely E, Westbrook D. Education of physicians-in-training can decrease the risk for vascular catheter infection. Ann Intern Med 2000; 132: 641-648.
- 8. O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recomm Rep 2002; 51 (RR-10): 1-29.
- 9. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU [erratum in N Engl J Med 2007; 356: 2660]. N Engl J Med 2006; 355: 2725-2732.
- 10. Clinical Excellence Commission. Central line associated bacteraemia in NSW intensive care units (CLAB ICU) final report. Sydney: CEC, 2010. http://www.cec.health.nsw.gov.au/__data/assets/pdf_file/0004/136552/final-report.pdf (accessed May 2011).
- 11. New South Wales Clinical Excellence Commission and Intensive Care Coordination and Monitoring Unit. Draft central line insertion and post insertion care guidelines. http://intensivecare.hsnet.nsw.gov.au/five/doc/statewide/draft_central_line_version1.2.pdf (accessed May 2011).
- 12. The Central Line Associated Bacteraemia in NSW Intensive Care Units (CLAB ICU) Collaborative. Central venous catheter insertion checklist. http://www.cec.health.nsw.gov.au/__data/assets/pdf_file/0007/135646/clabicuchecklist.pdf (accessed May 2011).
- 13. McLaws ML, Taylor PC. The Hospital Infection Standardised Surveillance (HISS) programme: analysis of a two-year pilot. J Hosp Infect 2003; 53: 259-267.
- 14. Centers for Disease Control and Prevention. Reduction in central line-associated bloodstream infections among patients in intensive care units – Pennsylvania, April 2001 – March 2005. MMWR Morb Mortal Wkly Rep 2005; 54: 1013-1016.
- 15. Koll BS, Straub TA, Jalon HS, et al. The CLABs collaborative: a regionwide effort to improve the quality of care in hospitals. Jt Comm J Qual Patient Saf 2008; 34: 713-723.
- 16. Bonello R, Fletcher C, Becker W, et al. An intensive care unit quality improvement collaborative in nine Department of Veterans Affairs Hospitals: reducing ventilator-associated pneumonia and catheter-related bloodstream infection rates. Jt Comm J Qual Patient Saf 2008; 34: 639-645.
- 17. Nolan T, Berwick D. All-or-none measurement raises the bar on performance JAMA 2006; 295: 1168-1170.
- 18. Munoz P, Bouza E, San Juan R, et al. Co-operative Group of the European Study Group on Nosocomial Infections (ESGNI). Clinical-epidemiological characteristics and outcome of patients with catheter-related bloodstream infections in Europe (ESGNI-006 Study) [erratum appears in Clin Microbiol Infect 2005; 11 Suppl 4: 65-68]. Clin Microbiol Infect 2004; 10: 843-845.
- 19. Gowardman JR, Robertson IK, Parkes S, et al. Influence of insertion site on central venous catheter colonization and bloodstream infection rates. Intensive Care Med 2008; 34: 1038-1045.
- 20. Lorente L, Jimenez A, Garcia C, et al. Catheter-related bacteremia from femoral and central internal jugular venous access. Eur J Clin Microbiol Infect Dis 2008; 27: 867-871.
- 21. Ishizuka M, Nagata H, Takagi K, et al. Femoral venous catheterization is a major risk factor for central venous catheter-related bloodstream infection. J Invest Surg 2009; 22: 16-21.
- 22. Posa PJ, Harrison D, Vollman KM. Elimination of central line-associated blood stream infections: application of the evidence. AACN Adv Crit Care 2006; 17: 446-454.
- 23. Carrer S, Bocchi A, Bortolotti M, et al. Effect of different sterile barrier precautions and central venous catheter dressing on the skin colonization around the insertion site. Minerva Anestesiol 2005; 71: 197-206.
- 24. Hu KK, Veenstra DL, Lipsky BA, Saint S. Use of maximal sterile barriers during central venous catheter insertion: clinical and economic outcomes. Clin Infect Dis 2004; 39: 1441-1445.
- 25. Hewson-Conroy K, Burrell A. Preliminary insights into the quality of data collected in NSW intensive care units. Crit Care Resusc 2008; 10: 301-305.
- 26. Drennan K, Hicks P, Hart GK. Australian and New Zealand Intensive Care Society. Centre for Outcome and Resource Evaluation. Intensive care safety and quality survey: Australia and New Zealand 2007/2008. Melbourne: ANZICS, 2010. http://www.anzics.com.au/core/reports (accessed May 2011).





Abstract
Objective: To reduce the rate of central line-associated bacteraemia (CLAB).
Design: A collaborative quality improvement project in intensive care units (ICUs) to promote aseptic insertion of central venous lines (CVLs). A checklist was used to record compliance with all aspects of aseptic CVL insertion, with maximal sterile barrier precautions for clinicians (“clinician bundle”) and patients (“patient bundle”). CLAB was identified and reported using a standard surveillance definition.
Participants and setting: Patients and clinicians in 37 ICUs in New South Wales, July 2007 – December 2008.
Main outcome measures: Compliance with aseptic CVL insertion; rates of CLAB.
Results: 10 890 CVL checklists were reviewed for compliance with the clinician and patient bundles: compliance with aseptic CVL insertion improved significantly (P < 0.001). The CLAB rate dropped from 3.0 to 1.2 per 1000 line-days (P < 0.001). Regardless of CVL type, the relative risk (RR) of CLAB in patients with CVLs inserted by clinicians not compliant with the clinician bundle was 1.62 times greater (95% CI, 1.1–2.4; P = 0.018) than the RR with CVLs inserted by clinicians compliant with both bundles. Compliance with both the bundles was associated with a 50% reduction in risk of CLAB (RR, 0.5; 95% CI, 0.4–0.8; P = 0.004).
Conclusions: Compliance with all aspects of aseptic CVL insertion significantly reduces the risk of CLAB. A difficulty we experienced was that most ICUs lacked the organisation and staff to support quality improvement and audit.