Sepsis (an acute infection with a systemic response) and severe sepsis (sepsis resulting in organ dysfunction) are very costly and often fatal conditions,1 and their incidence is increasing.2 Severe sepsis has been estimated to cause as many deaths annually in the United States as acute myocardial infarction.1 The epidemiology of sepsis has been well described in the US1,2 and Europe,3,4 but not in tropical regions or for indigenous populations. The one Australian study of sepsis epidemiology5 did not include data from tropical areas of Australia or on Indigenous status. This study found that the population-based incidence of severe sepsis requiring admission to an intensive care unit (ICU) was 0.77 cases per 1000 per year,5 which is comparable with figures reported from Europe and North America.6
The tropical Top End of the Northern Territory of Australia has a high proportion of Indigenous people, and the NT population has a high prevalence of infectious7 and chronic diseases;8 however, the epidemiology of sepsis in this population is unknown. Similar to many other indigenous peoples, Australia’s Indigenous population has a lower life expectancy and a higher burden of chronic and infectious diseases than non-Indigenous Australians.9
Most large studies of sepsis epidemiology are retrospective database analyses based on discharge coding,1,2,10 which is likely to significantly underestimate sepsis incidence.11 Most prospective studies are limited to patients requiring ICU admission,3,5 and are thus not representative of the true community burden of sepsis requiring hospitalisation. Patients with sepsis that requires hospital treatment, but not ICU admission, are common and have a high mortality rate,1 but these patients are under-represented in the medical literature.
Royal Darwin Hospital (RDH) is a 350-bed teaching hospital in Darwin (latitude 12.5°S) in the tropical Top End of the NT. RDH is the only hospital for a population of 130 000 over an area of 115 000 km2 (primary catchment area), and serves as a referral hospital (including for ICU admission) for a total population of 170 000 over an area of 500 000 km2. Indigenous Australians comprise 27% of the catchment population, and 30.3% of the population live in “remote” or “very remote” areas.12
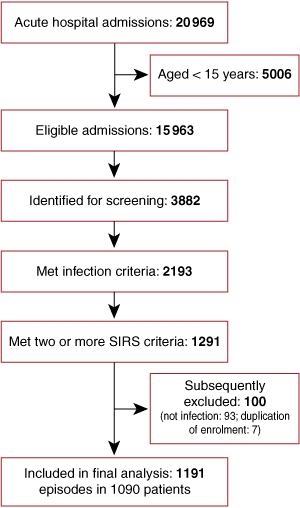
All patients who met predefined criteria for probable or definite infection (see Definitions), in addition to at least two criteria for the systemic inflammatory response syndrome (SIRS),13 were enrolled in the study. SIRS criteria (see Definitions) were required to be present concurrently within a 24-hour period, within the first 48 hours of hospital admission. Patients’ discharge summaries and pathology results were assessed at the time of hospital discharge, and those with a non-infectious cause of SIRS were subsequently excluded. A subset of 184 patients from this cohort who had pneumonia have been previously reported as part of an evaluation of pneumonia scoring systems.14
Criteria for probable or definite infection were those used in the PROWESS (Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis) study;15 ie, proven infection, or suspected infection, as evidenced by one or more of the following: (i) white cells in a normally sterile body fluid; (ii) perforated viscus; (iii) radiographic evidence of pneumonia in association with the production of purulent sputum; (iv) a syndrome associated with a high risk of infection (eg, ascending cholangitis); and (v) a visible site of infection (eg, cellulitis, abscess).
Severe sepsis was defined as sepsis plus at least one sepsis-related organ dysfunction within the first 48 hours after admission, as defined in the PROWESS study.15
After hand-checking of all case record forms, and data entry (Epidata 3.1, EpiData Foreningen, Odense, Denmark), 10% of entries were checked for errors, with a resulting error rate of < 0.1% of fields. Denominators for population-based incidence calculations were taken from the Australian Bureau of Statistics (ABS) estimated population figures for June 2007.16 Sepsis incidence was only calculated for patients whose current residence was within the primary catchment area of RDH; the incidence of severe sepsis requiring ICU admission was calculated using the primary catchment area for the ICU, a significantly larger area. Age-adjusted rates were calculated by the direct method, against the 2001 Standard Australian Population.17 Comparator studies of sepsis epidemiology were included if they used a similar methodology and reported comparable data to those reported in our study.
Indigenous population estimates were taken from ABS data from the 2006 census.12 Confidence intervals for age-adjusted rates were calculated using the Poisson distribution; P values of < 0.05 were considered significant. All statistical calculations were performed using Stata v10 (StataCorp, College Station, Tex, USA).
There were 1191 hospital admissions for community-onset sepsis in 1090 patients over the 12-month period (Box 1): patients’ mean age was 46.7 years, 52.4% were male, and 50.7% were Indigenous. The Indigenous population differed substantially from the non-Indigenous population in demographic characteristics, comorbidities, and alcohol and tobacco use (Box 2).
The overall population-based, age-adjusted incidence (95% CI) of sepsis was 11.8 (11.0–12.5) admissions per 1000 population per year compared with 40.8 (37.1–44.5) in Indigenous people (Box 3, A). The rates for severe sepsis requiring admission to the ICU were 1.3 (1.1–1.5) admissions per 1000 population per year overall and 4.7 (3.8–5.7) for Indigenous people (Box 3, B).
A causative organism was identified in 541 episodes of sepsis (45.4%); Staphylococcus aureus was the most common causative organism and Escherichia coli was the most common blood isolate (Box 4). Overall, the most common focus of infection was skin and soft tissue (32.8%) and, among those with severe sepsis, pneumonia was most common (44.8%).
Severity and outcome measures in patients with severe and non-severe sepsis, including hospital- and 28-day mortality rates, are compared in Box 5. Mortality rates were low by Australian and international standards, with 28-day mortality rates for sepsis, severe sepsis and severe sepsis requiring ICU admission of 5.4%, 17.1% and 21.5%, respectively; the corresponding mortality rates in the Indigenous subgroup were 5.7%, 15.9% and 19.3%. There was no significant difference in mortality rates between remote-dwelling (6.7%) and urban-dwelling (5.0%) patients.
On multivariate analysis, the strongest independent predictors of 28-day mortality rate were: older age, living in residential care, the number of SIRS criteria met during the first 48 hours of hospitalisation, and a serum albumin level on admission of < 35 g/L (Box 6). The crude and age-adjusted population-based sepsis mortality rates were 44.57 deaths per 100 000 per year and 80.33 deaths per 100 000 per year, respectively.
Compared with temperate areas of Australia,5 and the United Kingdom4 and France,22 the Top End has a significantly higher population-based incidence of severe sepsis requiring ICU admission, but this difference was entirely accounted for by the extremely high incidence in Indigenous people (Box 3, B). The rates of sepsis were about six times higher in our study than those reported from Victoria, a temperate region of Australia,21 and the US2 (Box 3, A), and much of this difference also derives from the rates in Indigenous people.
Patients in tropical NT with severe sepsis requiring admission to the ICU were younger than comparable patients in temperate Australia and had a lower 28-day mortality rate despite similar APACHE II and SOFA (sequential organ failure assessment) scores. Among those with severe sepsis, the two most common causative organisms (S. aureus and E. coli) were the same as those found in studies in temperate Australia,5 Canada25 and Europe,3 but there were significantly more gram-negative bacteria and fewer fungi among the causative organisms found in our study than in each of the other studies.
It is unclear why the incidence of sepsis is fourfold higher in Indigenous than non-Indigenous people. The design of our study did not allow us to determine community-based risk factors for sepsis; however, Indigenous people in our study had an excess of multiple comorbidities, which have been previously shown to increase the risk of sepsis or severe infections. These include diabetes, excessive alcohol use, chronic liver disease and end-stage renal disease. Other factors that are likely to contribute to the high burden of sepsis in Indigenous people include poor housing with a lack of health hardware (eg, water and sewerage)26 and overcrowding.27
There are no previous published studies describing the population-based epidemiology of sepsis in predominantly indigenous populations. However, high rates of infectious morbidity have been reported in indigenous populations in North America, Australia and New Zealand.9 The high rate of sepsis found in our study may reflect the high incidence of infections in Indigenous people rather than a tendency to develop sepsis in response to infection, but this hypothesis remains to be tested.
In our study, the relatively low mortality rate in patients admitted to the ICU for severe sepsis (21.5%) is consistent with mortality rates previously reported in patients with severe sepsis from RDH ICU (21%–25%),28,29 and is lower than the predicted mortality rate based on this cohort’s median APACHE II score (25.6%). This may be explained by the younger population in tropical NT compared with temperate Australia and elsewhere; if so, similar APACHE II scores, despite younger age, imply either more severe physiological disturbance or more comorbidities in our study population compared with other populations.
We cannot exclude the possibility that the higher incidence of sepsis in the Top End of the NT compared with incidence estimates from elsewhere reflects methodological differences. The comparator studies for sepsis incidence were all retrospective and based on discharge coding,2,10,21 a study design that is likely to substantially underestimate the true incidence of sepsis.11 This may explain why we found high rates of sepsis, but not of severe sepsis requiring ICU admission, in non-Indigenous people. The comparator study for severe sepsis requiring ICU admission in temperate Australia was prospective,5 and used very similar inclusion criteria and definitions, suggesting that the observed difference in incidence in the two studies is a true phenomenon. This emphasises that the primary finding of our study is the high rate of sepsis in Indigenous people, rather than in residents of the tropical NT in general.
3 Population-based incidence of sepsis requiring hospital admission (A) and severe sepsis requiring ICU admission (B) in the tropical Top End of the Northern Territory compared with other regions*
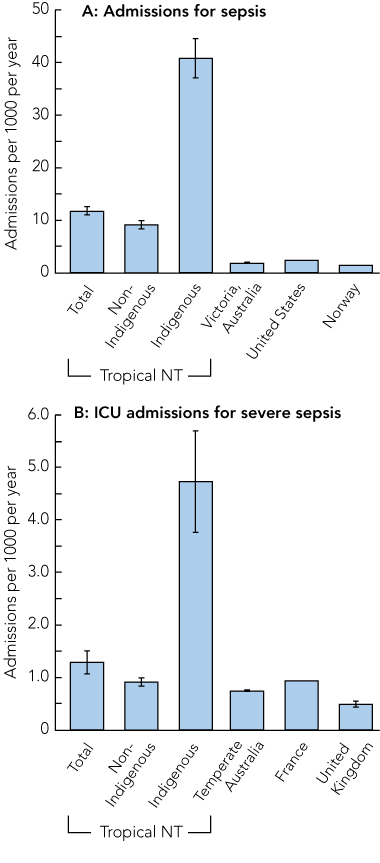
ICU = intensive care unit.
* Data are age-adjusted number of incident cases per 1000 population per year. Vertical lines at the top of each bar represent 95% CIs, where available. Data sources: Victoria, Australia;21 United States;2 Norway;10 temperate Australia;5 France;22 United Kingdom.4
5 Severity and outcome of sepsis and severe sepsis episodes*
6 Risk factors for 28-day mortality on univariate and multivariate analysis,* grouped according to the PIRO system
Provenance: Not commissioned; externally peer reviewed.
- Joshua S Davis1,2
- Allen C Cheng2,3,4
- Mark McMillan2
- Alex B Humphrey2
- Dianne P Stephens1
- Nicholas M Anstey1,2
- 1 Royal Darwin Hospital, Darwin, NT.
- 2 Global Health Division, Menzies School of Health Research and Charles Darwin University, Darwin, NT.
- 3 Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, VIC.
- 4 Alfred Hospital, Melbourne, VIC.
We would like to thank Bart Currie, John Condon, Steven Guthridge and Ric Price for advice regarding data collection and analysis; and Luke Diolosa for providing APACHE scores for patients admitted to the ICU. The study was funded by the National Health and Medical Research Council of Australia (Program Grants 290208, 496600; a Practitioner Fellowship to Nicholas Anstey, a Research Training Fellowship to Allen Cheng, and a PhD Scholarship to Joshua Davis).
None identified.
- 1. Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29: 1303-1310.
- 2. Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348: 1546-1554.
- 3. Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 2006; 34: 344-353.
- 4. Padkin A, Goldfrad C, Brady AR, et al. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Crit Care Med 2003; 31: 2332-2338.
- 5. Finfer S, Bellomo R, Lipman J, et al. Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Med 2004; 30: 589-596.
- 6. French CJ. The epidemiology of sepsis — is Australasia different? Crit Care Resusc 2006; 8: 219-222.
- 7. Blumer C, Roche P, Spencer J, et al. Australia’s notifiable diseases status, 2001: annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell 2003; 27: 1-78.
- 8. Zhao Y, Connors C, Wright J, et al. Estimating chronic disease prevalence among the remote Aboriginal population of the Northern Territory using multiple data sources. Aust N Z J Public Health 2008; 32: 307-313.
- 9. Gracey M, King M. Indigenous health part 1: determinants and disease patterns. Lancet 2009; 374: 65-75.
- 10. Flaatten H. Epidemiology of sepsis in Norway in 1999. Crit Care 2004; 8: R180-R184.
- 11. Ollendorf DA, Fendrick AM, Massey K, et al. Is sepsis accurately coded on hospital bills? Value Health 2002; 5: 79-81.
- 12. Australian Bureau of Statistics. Northern Territory — SSD by Indigenous status and age, from 2006 census of population and housing. Canberra: ABS, 2007.
- 13. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians / Society of Critical Care Medicine. Chest 1992; 101: 1644-1655.
- 14. Davis JS, Cross GB, Charles PG, et al. Pneumonia risk stratification in tropical Australia: does the SMART-COP score apply? Med J Aust 2010; 192: 133-136. <MJA full text>
- 15. Bernard GR, Vincent JL, Laterre PF, et al; Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) Study Group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001; 344: 699-709.
- 16. Australian Bureau of Statistics. Population by age and sex, Australian states and territories, Jun 2007. Canberra: ABS, 2007. (ABS Cat. No. 3201.0.)
- 17. Australian Bureau of Statistics. Australia’s standard population. Australian demographic statistics, September quarter 2002. Canberra: ABS. 2003. (ABS Cat. No. 3101.0.) http://www.aihw.gov.au/publications/aus/ah08/ah08-x02.rtf (accessed Feb 2011).
- 18. Australian Department of Health and Aged Care. Measuring remoteness: Accessibility / Remoteness Index of Australia (ARIA). Canberra: Commonwealth of Australia, 2001. http://www.health.gov.au/internet/main/publishing.nsf/Content/7B1A5FA525DD0D39CA2574 8200048131/$File/ocpanew14.pdf (accessed Feb 2011).
- 19. National Health and Medical Research Council. Australian alcohol guidelines: health risks and benefits. Canberra: NHMRC, 2001.
- 20. Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245-1251.
- 21. Sundararajan V, Macisaac CM, Presneill JJ, et al. Epidemiology of sepsis in Victoria, Australia. Crit Care Med 2005; 33: 71-80.
- 22. Brun-Buisson C, Meshaka P, Pinton P, et al. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Med 2004; 30: 580-588.
- 23. Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002; 137: 791-797.
- 24. Rubulotta F, Marshall JC, Ramsay G, et al. Predisposition, insult/infection, response, and organ dysfunction: a new model for staging severe sepsis. Crit Care Med 2009; 37: 1329-1335.
- 25. Martin CM, Priestap F, Fisher H, et al. A prospective, observational registry of patients with severe sepsis: the Canadian Sepsis Treatment and Response Registry. Crit Care Med 2009; 37: 81-88.
- 26. Torzillo PJ, Pholeros P, Rainow S, et al. The state of health hardware in Aboriginal communities in rural and remote Australia. Aust N Z J Public Health 2008; 32: 7-11.
- 27. McDonald MI, Towers RJ, Andrews RM, et al. Low rates of streptococcal pharyngitis and high rates of pyoderma in Australian Aboriginal communities where acute rheumatic fever is hyperendemic. Clin Infect Dis 2006; 43: 683-689.
- 28. Stephens DP, Thomas JH, Higgins A, et al. Randomized, double-blind, placebo-controlled trial of granulocyte colony-stimulating factor in patients with septic shock. Crit Care Med 2008; 36: 448-454.
- 29. Davis JS, Yeo TW, Thomas JH, et al. Sepsis-associated microvascular dysfunction measured by peripheral arterial tonometry: an observational study. Crit Care 2009; 13: R155.





Abstract
Objective: To describe the clinical and epidemiological features of sepsis and severe sepsis in the population of the tropical Top End of the Northern Territory of Australia and compare these with published estimates for temperate Australia, the United States and Europe.
Design, setting and participants: Prospective cohort study in the major hospital for tropical NT, a region where 27% of the population are Indigenous. We screened all adult (≥ 15 years) acute hospital admissions over a 12-month period (6 May 2007 – 5 May 2008) for sepsis by standard criteria, and collected standardised clinical data.
Main outcome measures: Population-based incidence of community-onset sepsis and severe sepsis requiring intensive care unit (ICU) admission; 28-day mortality rate and microbial epidemiology.
Results: There were 1191 hospital admissions for sepsis in 1090 patients, of which 604 (50.7%) were Indigenous people; the average age was 46.7 years. The age-adjusted annual population-based incidence of sepsis was 11.8 admissions per 1000 (mortality rate, 5.4%), but for Indigenous people it was 40.8 per 1000 (mortality rate, 5.7%). For severe sepsis requiring ICU admission, the incidence was 1.3 per 1000 per year (mortality rate, 21.5%), with an Indigenous rate of 4.7 per 1000 (mortality rate, 19.3%).
Conclusions: The incidence of sepsis in the tropical NT is substantially higher than that for temperate Australia, the United States and Europe, and these differences are mainly accounted for by the high rates of sepsis in Indigenous people. The findings support strategies to improve housing and access to health services, and reduce comorbidities, alcohol and tobacco use in Indigenous Australians. The burden of sepsis in indigenous populations worldwide requires further study to guide appropriate resourcing of health care and preventive strategies.