Chlamydial infection caused by Chlamydia trachomatis is the most prevalent sexually transmissible bacterial infection in the Western world1 and the most common notifiable infectious disease in Australia.2 In 2009, 62 680 chlamydia diagnoses were reported, with about 80% of these notifications being among young people aged 15–29 years3 — the age group with the highest documented chlamydia prevalence in Australia, with rates of at least 3%–5%.4,5
As chlamydia is mostly asymptomatic,6 regular testing is considered a key public health control strategy;2 if left untreated, infection may have significant clinical consequences such as pelvic inflammatory disease, infertility and pregnancy-related complications.6
Current national guidelines for general practitioners recommend annual screening for chlamydia for all sexually active people aged 15–25 years and those of any age reporting a recent partner change or inconsistent condom use.7 Other clinical guidelines extend testing to the age of 29 years among those who are sexually active.8 A recent mathematical modelling study found that annual chlamydia testing coverage rates of 20% in people aged under 30 years or 40% in those aged under 25 years would halve the prevalence in Australia within 4 years.9
Currently, there is no Australian information on the proportion of 15–29-year-olds tested for chlamydia by GPs. We assessed GP chlamydia testing rates in Australia through analysis of 12 months of Medicare data compiled as part of the Australian Collaboration for Chlamydia Enhanced Sentinel Surveillance (ACCESS; http://www.access-study.org).
All outcomes were analysed by patient age group, sex and area of residence (metropolitan or non-metropolitan, and state/territory). Metropolitan was defined by the Australian Bureau of Statistics (ABS) Australian Standard Geographical Classification – Remoteness Areas (ASGC-RA)10 as RA 0, and non-metropolitan as RA 1–4 using 2006 concordance data provided by the ABS. Under this classification, the capital cities of the Northern Territory (Darwin) and Tasmania (Hobart) are considered non-metropolitan.
The proportion of the 16–29-year-old population who had a GP consultation in the 12-month period was calculated using unique Medicare-rebated claims for consultations as the numerator, and the 2008 ABS Australian estimated resident population (ERP)11 of 16–29-year-olds as the denominator.
The annual GP chlamydia testing rate per 100 sexually active people aged 16–29 years was estimated using Medicare-rebated unique chlamydia tests as the numerator, and adjusted population denominators to reflect only the sexually active population. For analysis by state/territory and sex, the adjusted denominator was the 2008 ERP, and for rates by remoteness category the adjusted denominator was 2006 census data. Estimates from the Australian Study of Health and Relationships12 were used to calculate the proportion of sexually active 16–19-year-olds (male, 66%; female, 56%), 20–24-year-olds (male, 89%; female, 90%) and 25–29-year-olds (male, 95%; female, 97%). These proportions were applied to the 2008 ERP and 2006 census population figures to produce the adjusted denominators.
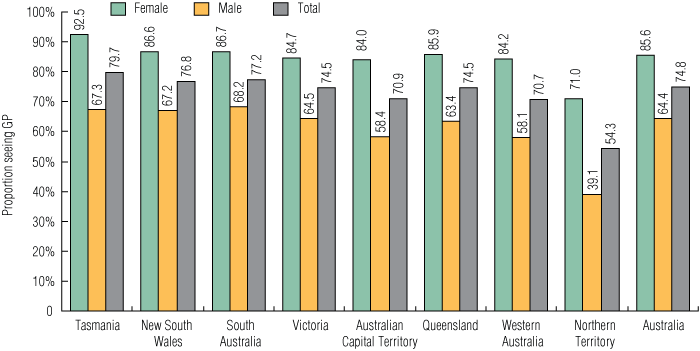
In 2008, the estimated total population of 16–29-year-olds in Australia was 4 195 961. During the study period, 3 139 354 unique GP consultations for 16–29-year-olds were reimbursed by Medicare nationally, meaning that 74.8% of this age group (85.6% of females and 64.4% of males) had at least one GP consultation in the 12-month period. The proportion of the population who had at least one GP consultation was lowest in the NT (54.3%) and highest in Tasmania (79.7%) (Box 1).
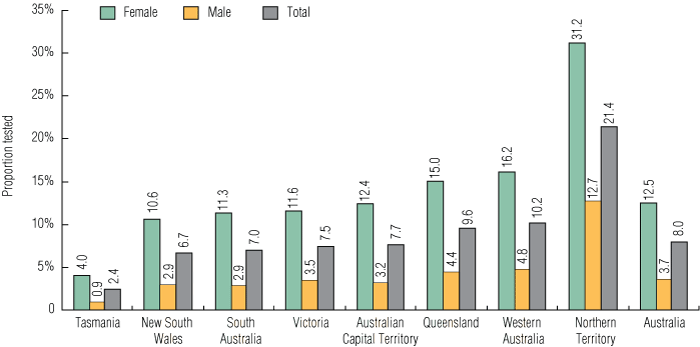
An estimated 8.0% of 16–29-year-olds in the sexually active Australian resident population had a chlamydia test reimbursed by Medicare during the study period. The testing rate per 100 sexually active population was higher for females (12.5%) compared with males (3.7%) (P < 0.01); higher in 20–24-year-olds (9.0%) compared with 16–19-year-olds (8.7%) and 25–29-year-olds (6.6%) (P < 0.01); higher in those living in non-metropolitan areas (11.0%) compared with metropolitan areas (8.4%) (P < 0.01); and highest in the NT (21.4%) and lowest in Tasmania (2.4%) (P < 0.01) (Box 2, Box 3).
Previous analyses have estimated GP chlamydia testing rates using population figures rather than patient consultations as the denominator and have only been conducted in Victoria, New South Wales and Tasmania.13-16 Furthermore, the rates found in those studies were considerably lower than those reported here, as the estimates were not adjusted for sexual activity in the populations studied.
Low testing rates may be due to sexual health forming only a small part of a GP’s workload,17 lack of time and knowledge about chlamydia and its risk factors, and patient embarrassment.18 GPs are more likely to test patients who report symptoms or a recent risk event; data from a survey of Victorian GPs19 and from 27 ACCESS general practice sentinel sites have demonstrated this preferential testing. The ACCESS study has found rates of chlamydia positivity about double (9.9% in males and 7.0% in females) that of the chlamydia prevalence estimates reported from community-based studies.4,5
Of all jurisdictions, the NT had the highest testing rate of 16–29-year-olds in the sexually active population and the lowest proportion of this age group who had visited a GP in the 12-month period. The reasons for this are not clear. It is possible that not all GP consultations were reflected in the data or that health care-seeking behaviour was systematically different. Higher testing rates may reflect the targeted screening programs for sexually transmitted infections conducted by government and Aboriginal medical services in the NT20 or a higher-risk population presenting to GPs. It is also unclear why Tasmania had the lowest testing rates, and this warrants further investigation.
Second, “remoteness” may be misclassified if testing is claimed by urban pathology providers for tests conducted on patients residing in non-urban areas. Furthermore, in our analysis, we used the ASGC-RA classification to assess remoteness, which classifies Hobart and Darwin as non-metropolitan, thus precluding meaningful assessment of remoteness as a factor in Tasmania and the NT. Future analyses should consider using the Accessibility/Remoteness Index of Australia classification scheme or McGrail’s index of rural access to ensure Darwin and Hobart are classified as urban.21
Despite these limitations, chlamydia testing rates based on Medicare data provide an important monitoring and evaluation indicator for public health policymakers to assess the impact of local, state and national initiatives such as the Australian Chlamydia Control Effectiveness Pilot.22 Testing rates based on the sexually active population also provide a more appropriate indicator to assess compliance with the clinical guidelines.
1 Proportion of the Australian estimated resident population aged 16–29 years with at least one general practitioner consultation, by sex and residence, October 2007 – September 2008
2 General practitioner chlamydia testing rate in 16–29-year-olds per 100 sexually active population, by sex and residence, October 2007 – September 2008
- Fabian Y S Kong1
- Rebecca J Guy2
- Jane S Hocking3
- Tony Merritt4
- Marie Pirotta5
- Clare Heal6
- Isabel Bergeri1
- Basil Donovan2
- Margaret E Hellard1
- 1 Centre for Population Health, Burnet Institute, Melbourne, VIC.
- 2 National Centre in HIV Epidemiology and Clinical Research, Sydney, NSW.
- 3 Centre for Women’s Health, Gender and Society, University of Melbourne, Melbourne, VIC.
- 4 Hunter New England Population Health, Hunter New England Area Health Service, Newcastle, NSW.
- 5 Department of General Practice and Primary Health Care Academic Centre, University of Melbourne, Melbourne, VIC.
- 6 Discipline of General Practice and Rural Medicine, James Cook University, Mackay, QLD.
The ACCESS project is funded by the Australian Government Department of Health and Ageing as part of the Chlamydia Targeted Grants Program. Fabian Kong managed the general practice network of the ACCESS project, and Margaret Hellard is one of the project’s Chief Investigators. The National Centre in HIV Epidemiology and Clinical Research is funded by the Australian Government Department of Health and Ageing.
None identified.
- 1. World Health Organization Department of HIV/AIDS. Global prevalence and incidence of selected curable sexually transmitted infections. http://www.who.int/docstore/hiv/GRSTI/003.htm (accessed Jan 2010).
- 2. Australian Government Department of Health and Ageing. National Sexually Transmissible Infections Strategy 2005–2008. Canberra: Commonwealth of Australia, 2005. http://www.health.gov.au/internet/main/publishing.nsf/Content/0333DF52D0E 2F3EDCA25702A0025132F/$File/sti_strategy.pdf (accessed Feb 2011).
- 3. Australian Government Department of Health and Ageing. National Notifiable Diseases Surveillance System. Number of notifications of Chlamydial infection, Australia, 2009 by age group and sex. http://www9.health.gov.au/cda/Source/Rpt_5.cfm (accessed Jan 2010).
- 4. Vajdic CM, Middleton M, Bowden FJ, et al. The prevalence of genital Chlamydia trachomatis in Australia 1997–2004: a systematic review. Sex Health 2005; 2: 169-183.
- 5. Hocking JS, Willis J, Tabrizi S, et al. A chlamydia prevalence survey of young women living in Mel-bourne, Victoria. Sex Health 2006; 3: 235-240.
- 6. Peipert JF. Genital chlamydial infections. N Engl J Med 2003; 349: 2424-2430.
- 7. Harris M, Bennett J, Del Mar C, et al. Guidelines for preventive activities in general practice. 7th ed. Melbourne: Royal Australian College of General Practitioners, 2009.
- 8. Bourke S, editor. National management guidelines for sexually transmissible infections. 7th ed. Melbourne: Sexual Health Society of Victoria, 2008. http://www.mshc.org.au/Portals/6/NMGFSTI.pdf (accessed Feb 2011).
- 9. Regan DG, Wilson DP, Hocking JS. Coverage is the key for effective screening of Chlamydia trachomatis in Australia. J Infect Dis 2008; 198: 349-358.
- 10. Australian Bureau of Statistics. Australian Standard Geographical Classification (ASGC), 2005. Canberra: ABS, 2005. (ABS Cat. No. 1216.0.)
- 11. Australian Bureau of Statistics. Australian demographic statistics, Jun 2008. Canberra: ABS, 2008. (ABS Cat. No. 3101.0.)
- 12. de Visser RO, Smith AMA, Rissel CE, et al. Sex in Australia: heterosexual experience and recent heterosexual encounters among a representative sample of adults. Aust N Z J Public Health 2003; 27: 146-154.
- 13. Hocking J, Fairley C, Counahan M, Crofts N. The pattern of notification and testing for genital Chlamydia trachomatis infection in Victoria, 1998–2000: an ecological analysis. Aust N Z J Public Health 2003; 27: 405-408.
- 14. Chen MY, Fairley CK, Donovan B. Nowhere near the point of diminishing returns: correlations between chlamydia testing and notification rates in New South Wales. Aust N Z J Public Health 2005; 29: 249-253.
- 15. Stephens N, O’Sullivan M, Coleman D, Shaw K. Chlamydia trachomatis in Tasmania 2001–2007: rising notification trends. Aust N Z J Public Health 2010; 34: 120-125.
- 16. McNamee KM, Fairley CK, Hocking JS. Chlamydia testing and notification in Australia: more money, more tests. Sex Transm Infect 2008; 84: 565-569.
- 17. Johnston VJ, Britt H, Pan Y, Mindel A. The management of sexually transmitted infections by Australia general practitioners. Sex Transm Infect 2004; 80: 212-215.
- 18. Hocking JS, Parker RM, Pavlin N, et al. What needs to change to increase chlamydia screening in general practice in Australia? The views of general practitioners. BMC Public Health 2008; 8: 425.
- 19. Hocking JS, Lim MSC, Vidanapathirana J, et al. Chlamydia testing in general practice — a survey of Victorian general practitioners. Sex Health 2006; 3: 241-244.
- 20. Huang RL, Torzillo PJ, Hammond VA, et al. Epidemiology of sexually transmitted infections on the Anangu Pitjantjatjara Yankunytjatjara Lands: results of a comprehensive control program. Med J Aust 2008; 189: 442-445.
- 21. McGrail MR, Humphreys JS. Geographical classifications to guide rural health policy in Australia. Aust New Zealand Health Policy 2009, 6: 28.
- 22. Hocking JS, Temple-Smith M, Low N, et al. ACCEPT (Australian Chlamydia Control Effectiveness Pilot): design of the pilot evaluation [abstract]. In: Proceedings of the Australasian Sexual Health Conference; 2009 Sep 7–9; Brisbane, Qld. Sydney: Australasian Chapter of Sexual Health Medicine, Royal Australasian College of Physicians, 2009.





Abstract
Objective: To describe the proportion of 16–29-year-olds tested for chlamydia by Australian general practitioners in a 12-month period.
Design and setting: Between October 2007 and September 2008, the national chlamydia testing rate in 16–29-year-olds was calculated by dividing the number of Medicare-reimbursed chlamydia tests by two denominators: (i) Medicare-reimbursed GP consultations; and (ii) estimated resident populations adjusted for the proportion who were sexually active.
Main outcome measures: GP chlamydia testing rates in 16–29-year-olds per 100 patients attending a GP consultation and per 100 sexually active population, by patient age and sex, state/territory of residence, and remoteness area.
Results: Among the estimated Australian population of 16–29-year-olds, 85.6% of females and 64.4% of males had at least one GP consultation in the 12-month period. The national GP chlamydia testing rate per 100 patients was 8.9% (95% CI, 8.88%–8.94%). The national GP chlamydia testing rate per 100 sexually active population was 8.0% (95% CI, 7.92%–7.98%). The rate per 100 sexually active population was higher in females (12.5%) compared with males (3.7%) (P < 0.01); higher in 20–24-year-olds (9.0%) compared with 16–19-year-olds (8.7%) and 25–29-year-olds (6.6%) (P < 0.01); higher in those living in non-metropolitan areas (11.0%) compared with metropolitan areas (8.4%) (P < 0.01); and highest in those living in the Northern Territory (21.4%) compared with other jurisdictions (P < 0.01).
Conclusions: Despite clinical guidelines recommending annual chlamydia testing for sexually active 15–29-year-olds, our analysis showed that a high proportion of young people aged 16–29 years attend a GP each year, but few of the sexually active population in this age group were tested for chlamydia in general practice. Strategies are needed to support GPs to enhance chlamydia testing in young people.