Relationships between the pharmaceutical industry and medical schools have recently been increasingly drawn into the media spotlight because of their ability to create conflicts of interest. Much of this attention is due to evidence that industry relationships can influence the attitudes and behaviours of medical students, with effects persisting beyond graduation. A recent review by the American Medical Student Association (AMSA) of United States medical school policies highlights the lack of policies regarding disclosure and management of conflicts of interest between faculty members and industry.1 Furthermore, some medical schools even rely on industry for a significant portion of their operating budgets.2 This reliance can blur the distinction between the primary motive of universities to facilitate independent education and research and the primary motive of industry to generate profits for its shareholders.
Industry promotes itself and its products to academics, doctors in practice and medical students in a number of ways. These include the provision of gifts, drug samples, honoraria for research and speaking engagements, travel funding and payments for being on advisory boards. Conflicts may also arise from the provision of gifts or discretionary funding from industry to individual institutions.2,3 Medical students have been shown to be vulnerable to these influences, despite the fact that most believe they are personally immune.4-9 Indeed, as the attitudes and behaviour formed while in medical school have been shown to persist into professional life,10 it is important both to provide a detailed and balanced education to medical students about these relationships with industry and to protect students (and their future patients) from the sequelae of undue influence by industry.
Medical school policies regulating interaction with the pharmaceutical industry are effective in helping students to maintain a degree of independence from industry bias. Policies can temper a preference for brand-name medications over generics,11 reduce the likelihood of gifts being accepted,12 and lower future interactions with industry, including the receipt of consulting fees.7,12,13 Furthermore, the beneficial influence of these institutional policies on attitudes and behaviour persists after graduation.7 For an overview of recent and current US and Australian conflict-of-interest policy recommendations and regulation, see Box 1. Of particular note, in 2007, the AMSA released the PharmFree Scorecard (http://www.amsascorecard.org), which evaluates medical school policies for their ability to manage potential conflicts of interest.
The purpose of our study was to examine the adequacy of policies at Australian medical schools with respect to declaring and managing potential conflicts of interest with industry.
Permission was obtained from AMSA to use its PharmFree Scorecard as an assessment tool for our survey. In AMSA ’s 2009 survey of the conflict-of-interest policies of 150 US medical schools, the scorecard covered 11 domains (broadly consistent with those identified previously3).
In October 2009, we wrote to the deans of the 20 Australian medical schools, outlining the project and requesting submission of their policies. Following feedback from medical schools, we excluded four domains from our evaluation, as they did not reflect the Australian medical school environment. These domains were pharmaceutical samples, purchasing and formularies, industry sales representatives, and industry funding for trainees.
Consequently, we rated policies across seven domains: gifts, consulting relationships, industry-funded speaking relationships, disclosure, on-campus educational activities, travel to off-campus educational activities, and curriculum. Each domain was scored as follows:
3 = model policy
2 = good progress towards model policy
1 = policy absent or unlikely to have a substantial effect on behaviour.
The application of specific criteria during policy appraisal ensured that domains were scored objectively (Box 2).
Each policy was graded by two assessors, blinded to the institution of origin, applying the unique criteria for each domain. Progress results for each medical school were forwarded to that school for suggested amendments. Any differences in grading were resolved by a third, independent assessor.
The mean final score in Australia was compared with the mean score from the AMSA 2009 survey, by reanalysing the seven domains included in the Australian survey, using the two-sample t test. Medians were compared using the Wilcoxon rank-sum test.
Grades were assigned using the AMSA grading system: A ≥ 85%, B = 70%–84%, C = 60%–69%, D = 40%–59%, F < 40%, and in process. Non-reporting institutions received a domain score of 1 and a grade of F, in line with the AMSA methods.
Ethics approval was not sought for this study. As medical schools are public institutions that receive federal funding, it was not felt necessary to obtain ethics approval from the schools, as data sought were not of a personal or individual nature. Furthermore, most data were already available on publicly accessible websites. This was consistent with the methods applied by the AMSA. Institutions were advised in writing at the time of request that the results would form the basis of a thesis with the prospect of publication.
Of 20 medical schools surveyed, five reported having two or more policies in development (range, 3–7) and were graded as being in process (Box 3). Data for medical schools graded as being in process were not used in calculating mean scores. Nine medical schools were graded D, and five were graded F (three of which failed to provide any information). James Cook University’s medical school received the highest overall score of 67% and was graded C (Box 4).
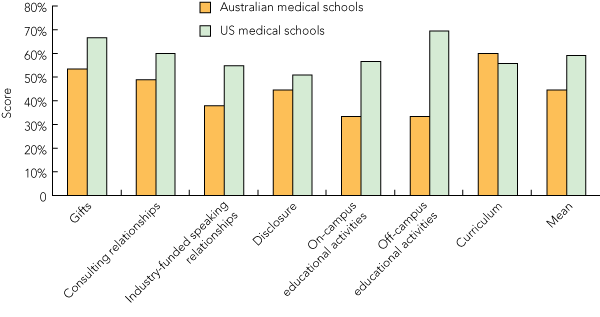
Across the seven domains assessed, curriculum received the highest mean score (60%), with seven medical schools achieving the maximum score of 3 (the University of Western Sydney scored 3 in this domain, but was excluded from calculation as it had four policies in process). Curriculum was the only domain in which any medical school scored 3. Gifts received the second highest mean score overall (53%). The domains with the lowest mean scores were on-campus educational activities and travel to off-campus educational activities. Of the 15 medical schools included in our mean domain score calculations, Bond University and James Cook University indicated that they had policies in process for the domain of on-campus educational activities. All other universities received the lowest score of 1 in these two domains.
The domains with the highest mean scores in the 2009 AMSA survey were off-campus educational activities, gifts and consulting relationships (Box 5). The number of perfect scores in 2009 was almost double that in AMSA’s 2008 survey. On-campus educational activities had the fewest perfect scores in AMSA’s 2009 survey — a finding consistent with our results for Australian medical schools.
Compared with the AMSA 2009 results, Australian medical schools performed better in only the curriculum domain (Box 5). The mean final score for Australian institutions was lower than that for US institutions (44% v 58%, P < 0.001; difference in mean scores, 14% [95% CI, 8%–20%]) (Box 6). The Australian median final score was also significantly lower than that for the US institutions (48% v 62%, P = 0.004).
Twenty-five per cent of Australian respondents reported two or more policies in process compared with 18% of AMSA 2009 survey respondents. A similar proportion of institutions in the US (16%) and Australia (15%) did not submit a response to each survey.
Overall, medical schools in the US have more robust policies governing potential conflicts of interest than their Australian counterparts. It is possible that the sequence of surveys stimulated policy improvements seen in US medical schools between 2008 and 2009. In our survey, seven of the 20 Australian medical schools reported policies in development. Furthermore, some schools requested a copy of our findings, suggesting a commitment to enhancing their policies.
A significant number of US medical schools are stand-alone institutions, whereas all Australian medical schools exist under the auspices of a particular university. Consequently, policies governing conflicts of interest at Australian medical schools may reflect general university policy and thus may not address the specific challenges of medical education and research. Consideration should be given to helping Australian medical schools to develop conflict-of-interest policies distinct from their parent university.
Our results indicate a need for improved self-regulation of conflicts of interest by Australian medical schools. Medical schools should continue to recognise their influential position in society, and be aware that their financial relationship with industry is an area in which they should demonstrate leadership in the quest towards the highest possible ethical standards in medical education and research. Failure to take this opportunity may not only compromise the standing of the medical profession in society and the quality of medical research and patient care, but may also lead to the imposition of legislative controls governing disclosure and management of conflicts of interest.
1 Conflict-of-interest policy recommendations and regulation in the United States and Australia
Policy reform in the US
In 2002, the Association of American Medical Colleges released policy recommendations regarding institutional conflicts of interest. Four years later, these policy recommendations had only been implemented by 38% of US medical colleges. In May 2007, the American Medical Student Association (AMSA), concerned at this inaction and the likely repercussions of uncontrolled and often hidden conflicts of interest within medical schools, released the PharmFree Scorecard (http://www.amsascorecard.org), evaluating medical school policies for their ability to manage potential conflicts of interest. The AMSA found that only 21 of 150 medical schools had adequate policies. Significant media attention focused on these findings, and reports of Harvard Medical School students protesting against their school’s poor showing made international news.
In January 2009, the Physician Payments Sunshine Bill was introduced in the US Congress with the stated intent of making relationships between physicians and industry transparent. On learning that 23 institutions had either declined or failed to respond to requests by AMSA for policy information, Senator Charles Grassley, one of the Bill’s co-sponsors, issued a public letter of reprimand to these institutions. He requested detailed data on industry funding and noted the National Institutes of Health requirement that institutions must manage conflicts of interest to be eligible to receive funding. Senator Grassley also made specific note of three Harvard medical professors who each failed to disclose about one million US dollars in outside income from industry. He sent a letter to Pfizer asking for detailed information about payments made to these and other Harvard medical faculty members. A congressional investigation into these payments found serious inconsistencies between the funding reported by industry and that declared by the faculty.
In 2009, a report from the Institute of Medicine called for a ban on all gifts from industry to medical school faculties.14,15 The Physician Payments Sunshine Act was recently passed into law in the US. This Act mandates that, from 1 January 2012, drug and medical device manufacturers must publicly report gifts and payments made to physicians and teaching hospitals.
The Australian context
While the voices calling for reform in Australia have not reached the volume of those in the US, interest in the issue is growing.
In 2006, the Australian Competition and Consumer Commission (ACCC) required that the pharmaceutical industry representative body, Medicines Australia, publish detailed costings of hospitality and entertainment at educational events directed at medical practitioners. The ACCC recently extended this condition to include events that are funded by members of the Generic Medicines Industry Association (GMIA) and directed at pharmacists. Furthermore, the ACCC now requires disclosure of the nature and value of any gifts provided to pharmacists by GMIA members. Pharma Phacts, a medical student-led organisation attempting to raise awareness of the industry influence on medical students, was launched in July 2009. In April 2010, the Australian Medical Association (AMA) released a revised position statement providing guidance to doctors on maintaining ethical relationships with industry.16
A recent review of the exposure of medical students to pharmaceutical industry promotion included 10 articles, but none addressed the Australian situation.9 The current regulatory environment is both complex and confusing. Medical students are covered by policies from their university and medical school as well as any hospitals with which they may be affiliated. Furthermore, the AMA position statement16 refers to medical students with respect to their ethical development and attendance at sponsored events. The pharmaceutical industry self-regulates its conduct through Medicines Australia, with the oversight of regulatory bodies such as the ACCC.
2 Domains and rating criteria for Australian medical schools’ conflict-of-interest policies*
Domain/ rating |
Criteria |
||||||||||||||
Gifts (including meals) |
|||||||||||||||
3 |
All gifts and on-site meals funded by industry are prohibited, regardless of nature or value. |
||||||||||||||
2 |
Less stringent limitation on industry-funded gifts (eg, gifts above $50 per year prohibited , or gifts prohibited but meals allowed). |
||||||||||||||
1 |
No policy, or policy that would not substantially reduce gifting (eg, gifts are allowed but discouraged, or limited in a non-specific way to “appropriate”, or primarily for the benefit of patients). |
||||||||||||||
Consulting relationships (excluding scientific research and speaking) |
|||||||||||||||
3 |
Consulting relationships with industry must be subjected to institutional review or approval. Additionally, they must either be described in a formal contract, or payment for services must be commensurate with the task. |
||||||||||||||
2 |
As above, without the institutional review or approval requirement. |
||||||||||||||
1 |
No policy, or policy that would allow consulting relationships to occur without institutional scrutiny or that would allow relationships in which payments are not commensurate with work. |
||||||||||||||
Industry-funded speaking relationships |
|||||||||||||||
3 |
Speaking relationships are prevented from functioning as de-facto gifts or marketing. An effective policy must not implicitly permit (a) long-term speaking agreements or (b) industry to have a role in determining presentation content. (Some effective policies may explicitly prohibit participation in a speakers bureau. Other effective policies contain elements such as limits on compensation and reimbursement, and a requirement to ensure the scientific integrity of information presented.) |
||||||||||||||
2 |
Industry-funded speaking relationships are regulated, but with less stringent limits on longevity, content or compensation. |
||||||||||||||
1 |
No policy, or policy that does not define the limits on longevity, content or compensation. |
||||||||||||||
Disclosure |
|||||||||||||||
3 |
Personnel are required to disclose past and present financial ties with industry (eg, consulting and speaking agreements, research grants) on a publicly available website and/or disclose such relationships to patients when such a relationship might represent an apparent conflict of interest. |
||||||||||||||
2 |
Universally required, internal disclosure to the medical school or hospital administration. (Policies requiring disclosure only when presenting or publishing do not meet this criterion.) |
||||||||||||||
1 |
No policy. |
||||||||||||||
On-campus educational activities |
|||||||||||||||
3 |
Industry is not permitted to provide direct financial support for educational activities, including continuing medical education, directly or through a subsidiary agency. (However, companies may contribute unrestricted funds to a central fund or oversight body at the academic medical centre, which, in turn, would pool and disburse funds for programs that are independent of any industry input or control.) |
||||||||||||||
2 |
Less stringent limitations to ensure independence of educational content (eg, standards to establish freedom from industry influence on content, such as review and approval of presentations; language that prevents industry from selecting the speaker). Industry funding may be allocated for a particular topic, but must be provided directly to the department, not to individuals. |
||||||||||||||
1 |
No policy, or a policy that would not substantially limit industry influence over educational activities (eg, industry funding must be disclosed). |
||||||||||||||
Travel to off-campus educational activities |
|||||||||||||||
3 |
Personnel may not accept payment, gifts or financial support from industry to attend lectures and meetings. (An exception may be made for modest meals, if part of a larger program.) Travel support may only be accepted if it is subject to institutional approval or industry is prevented from selecting the recipients. |
||||||||||||||
2 |
Less stringent limitations. |
||||||||||||||
1 |
No policy, or a policy that would not substantially limit participation in industry-funded events and meetings. |
||||||||||||||
Medical school curriculum (or other documentation of educational objectives or course content) |
|||||||||||||||
3 |
Students are trained to understand institutional conflict-of-interest policies and recognise how industry promotion can influence clinical judgement. |
||||||||||||||
2 |
Curriculum addresses conflict of interest in a more limited way (eg, training on policies only). |
||||||||||||||
1 |
No policy (not addressed in curriculum or elsewhere). |
||||||||||||||
* Source: http://www.amsascorecard.org/methodology. |
|||||||||||||||
3 Australian medical schools with ≥ 2 conflict-of-interest policies in process*
Medical school |
Gifts |
Consulting relationships |
Industry-funded speaking relationships |
Disclosure |
On-campus educational activities |
Off-campus educational activities |
Curriculum |
||||||||
University of Melbourne, Vic |
In process |
In process |
In process |
In process |
In process |
In process |
In process |
||||||||
University of New South Wales, NSW |
In process |
In process |
In process |
In process |
In process |
In process |
In process |
||||||||
University of Notre Dame (Fremantle), WA |
In process |
In process |
1 |
In process |
1 |
1 |
1 |
||||||||
University of Western Sydney, NSW |
In process |
In process |
In process |
In process |
1 |
1 |
3 |
||||||||
University of Wollongong, NSW |
In process |
In process |
In process |
In process |
In process |
In process |
In process |
||||||||
3 = model policy. 1 = policy is absent or unlikely to have a substantial effect on behaviour. NSW = New South Wales. Vic = Victoria. WA = Western Australia. |
|||||||||||||||
4 Conflict-of-interest policy scores and grades for Australian medical schools with ≤ 1 conflict-of-interest policy in process*
Medical school |
Gifts |
Consulting relationships |
Industry-funded speaking relationships |
Disclosure |
On-campus educational activities |
Off-campus educational activities |
Curriculum |
Mean overall score |
Grade† |
||||||
James Cook University, Qld |
2 |
2 |
2 |
2 |
In process |
1 |
3 |
67% |
C |
||||||
Australian National University, ACT |
1 |
2 |
1 |
2 |
1 |
1 |
3 |
52% |
D |
||||||
Monash University, Vic |
2 |
2 |
2 |
2 |
1 |
1 |
1 |
52% |
D |
||||||
University of Queensland, Qld |
2 |
2 |
1 |
1 |
1 |
1 |
3 |
52% |
D |
||||||
University of Newcastle, NSW |
2 |
1 |
1 |
1 |
1 |
1 |
3 |
48% |
D |
||||||
University of Sydney, NSW |
2 |
2 |
1 |
2 |
1 |
1 |
1 |
48% |
D |
||||||
University of Tasmania, Tas |
2 |
2 |
1 |
2 |
1 |
1 |
1 |
48% |
D |
||||||
University Western Australia, WA |
2 |
1 |
1 |
1 |
1 |
1 |
3 |
48% |
D |
||||||
Bond University, Qld |
2 |
2 |
1 |
1 |
In process |
1 |
1 |
44% |
D |
||||||
Flinders University, SA |
1 |
1 |
1 |
1 |
1 |
1 |
3 |
43% |
D |
||||||
University of New England, NSW |
2 |
1 |
1 |
1 |
1 |
1 |
1 |
38% |
F |
||||||
Griffith University, Qld |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
33% |
F |
||||||
Deakin University, Vic |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
33% |
F‡ |
||||||
University of Adelaide, SA |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
33% |
F‡ |
||||||
University of Notre Dame (Sydney), NSW |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
33% |
F‡ |
||||||
Mean domain score§ |
53% |
49% |
38% |
44% |
33% |
33% |
60% |
|
|
||||||
3 = model policy. 2 = good progress toward model policy. 1 = policy is absent or unlikely to have a substantial effect on behaviour. ACT = Australian Capital Territory. NSW = New South Wales. Qld = Queensland. SA = South Australia. Tas = Tasmania. Vic = Victoria. WA = Western Australia. * In process = policy currently in development. † C = mean overall score, 60%–69%; D = mean overall score, 40%–59%; F = mean overall score, < 40%. ‡ Failed to submit response to survey. § Mean domain score was calculated as cumulative score for each domain divided by maximum possible score. |
|||||||||||||||
5 Mean scores for United States and Australian medical schools’ conflict-of-interest policies, by domain
6 Management of conflict of interest in United States and Australian medical schools, by institutional mean overall scores
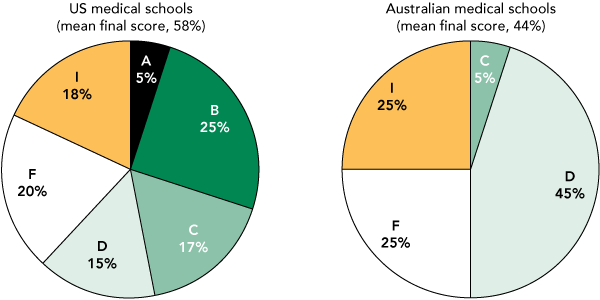 Institutional mean overall scores graded as follows: A ≥ 85%; B = 70%–84%; C = 60%–69%; D = 40%–59%; F < 40%; and I = in process. Institutions that failed to submit response to survey received a grade of F. |
Received 16 August 2010, accepted 18 October 2010
- Paul R Mason1
- Martin H N Tattersall2
- 1 Royal Melbourne Hospital, Melbourne, VIC.
- 2 Central Clinical School, University of Sydney and Royal Prince Alfred Hospital, Sydney, NSW.
We thank Dr Kevin McGeechan of the School of Public Health, University of Sydney, for his statistical expertise. We thank Fiona McQualter for her assistance in reviewing and scoring the institutional policies, and for reviewing the manuscript.
None identified.
- 1. Yager J, Feinstein RE. Medical education meets pharma: moving ahead. Acad Psychiatry 2010; 34: 92-97.
- 2. Campbell EG, Weissman JS, Ehringhaus SE, et al. Institutional academic industry relationships. JAMA 2007; 298: 1779-1786.
- 3. Brennan TA, Rothman DJ, Blank L, et al. Health industry practices that create conflicts of interest: a policy proposal for academic medical centers. JAMA 2006; 295: 429-433.
- 4. Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA 2000; 283: 373-380.
- 5. Sagarin BJ, Cialdini RB, Rice WE, et al. Dispelling the illusion of invulnerability: the motivations and mechanisms of resistance to persuasion. J Pers Soc Psychol 2002; 83: 526-541.
- 6. Norris P, Herxheimer A, Lexchin J, Mansfield P. Drug promotion: what we know, what we have yet to learn. Geneva: World Health Organization and Health Action International, 2005.
- 7. Zipkin DA, Steinman MA. Interactions between pharmaceutical representatives and doctors in training. A thematic review. J Gen Intern Med 2005; 20: 777-786.
- 8. Sierles FS, Brodkey AC, Cleary LM, et al. Medical students’ exposure to and attitudes about drug company interactions: a national survey. JAMA 2005; 294: 1034-1042.
- 9. Carmody DM, Mansfield PR. What do medical students think about pharmaceutical promotion? Aust Med Student J 2010; 1: 54-57.
- 10. McCormick B, Tomlinson G, Brill-Edwards P, Detsky A. Effect of restricting contact between pharmaceutical company representatives and internal medicine residents on posttraining attitudes and behavior. JAMA 2001; 286: 1994-1999.
- 11. Grande D, Frosch DL, Perkins AW, Kahn BE. Effect of exposure to small pharmaceutical promotional items on treatment preferences. Arch Intern Med 2009; 169: 887-893.
- 12. Gundermann C, Meier-Hellmann A, Bauer M, Hartmann M. [Effects of a mandatory guideline that prohibits hospital doctors from accepting any form of benefits in any form from the pharmaceutical industry] [German]. Dtsch Med Wochenschr 2010; 135: 67-70.
- 13. Moynihan R. Drug company sponsorship of education could be replaced at a fraction of its cost. BMJ 2003; 326: 1163.
- 14. Rothman DJ, McDonald WJ, Berkowitz CD, et al. Professional medical associations and their relationships with industry: a proposal for controlling conflict of interest. JAMA 2009; 301: 1367-1372.
- 15. Steinbrook R. Controlling conflict of interest — proposals from the Institute of Medicine. N Engl J Med 2009; 360: 2160-2163.
- 16. Australian Medical Association. Position statement on doctors’ relationships with industry 2010. Canberra: AMA, 2010. http://ama.com.au/node/5421 (accessed Dec 2010).





Abstract
Objective: To examine the adequacy of policies at Australian medical schools for managing potential conflicts of interest with the pharmaceutical industry.
Design, setting and participants: National survey of 20 Australian medical schools to assess their policies regarding disclosure and management of conflict of interest, undertaken in October 2009, using the American Medical Student Association’s PharmFree Scorecard.
Main outcome measures: Policy scores and grades for Australian medical schools.Results: Compared with United States medical schools, Australian medical schools performed better in only the curriculum domain and had a lower mean score overall (44% v 58%; P < 0.001).
Conclusion: Our results indicate a need for improved self-regulation of conflicts of interest in Australian medical schools.