Iodine deficiency has re-emerged in south-eastern Australia. Following reports of iodine deficiency in localised studies of children1 and pregnant women,1-5 the 2003–2004 Australian National Iodine Nutrition Study (NINS) found that primary school-aged children in the south-eastern states, but not Queensland or Western Australia, had median urinary iodine concentrations (MUICs) in the mild deficiency range.6 The Northern Territory was excluded from the NINS for logistical reasons, and Indigenous Australians were included in the study only to the extent that they were part of the general population. The re-emergence of iodine deficiency in Australia and its potential adverse effects on the developing brain have led to mandatory replacement of non-iodised salt with iodised salt in bread from October 2009,7 and a recommendation that pregnant and breastfeeding women consider taking a daily iodine supplement.8
The iodine status of populations is defined by measuring MUIC.9 The urinary excretion of iodine, in common with several other urinary electrolytes,10 reflects recent intake. As high concentrations of iodine are found in a limited range of foods, the iodine concentration in a spot urine sample, or even a 24-hour urine sample, may not reflect the long-term iodine intake, and therefore iodine status, of the individual. Within-person variation is a type of random error and, in a population survey, it increases the spread of a distribution.11 Consequently, a survey that collects a single urine sample from each subject can describe the average iodine level in the population, but other points on the distribution (eg, the 75th centile) reflect the value on the day of collection rather than long-term population values.12 Several methods exist to correct for day-to-day (within-person) variation in population survey data. One method is to collect a second set of data from a subset of the survey population to calculate a correction factor that is then applied to the main survey population distribution.7,10,13,14
Participants in our study were members of the Australian Aboriginal Birth Cohort, recruited at infancy in 1987–1990 and followed up in 2005–2008.15,16 We describe the iodine status of Aboriginal teenagers living in the Darwin Health Region (DHR), which covers 120 000 km2 of the “Top End” of the NT.
Subject recruitment and follow-up for the Aboriginal Birth Cohort Study are described in detail elsewhere.15,16 Briefly, 686 infants of women recorded as Aboriginal in the delivery suite register of the Royal Darwin Hospital between January 1987 and March 1990 were recruited. Apart from a small private hospital, this was the only hospital in the DHR at the time. Births in the hospital include all routine deliveries of infants from the DHR and high-risk deliveries referred from a larger area across northern Australia. Of the infants recruited to our study, 570 were from routine deliveries to mothers in the DHR. Recruitment depended on the availability of the neonatal paediatrician to see the mother. About half of all eligible infants were recruited and there was no difference in the birthweight distribution or sex ratio between those recruited and those not recruited.15
Participants were followed up between December 2005 and January 2008 (about 18 years after recruitment) in over 40 locations.16 A spot urine sample was collected in addition to height and weight measurements. Girls were asked if they were pregnant, as well as the number and age of any other children. Because the NT has a unique identification number for each person that is used in all hospitals, publicly funded health clinics and associated data collections, we used the NT Perinatal Data Collection as a supplementary source of information. Girls who stated that they were pregnant at the interview (n = 20) or had a birth recorded in the Perinatal Data Collection less than 9 months after the interview (n = 4) were classified as pregnant for the current analysis. The method of infant feeding was not ascertained, but breastfeeding is the normal practice in NT Aboriginal communities, so girls with an infant born less than 6 months before the date of interview (n = 11) were assumed to be breastfeeding and grouped separately from other non-pregnant girls. Girls who were neither pregnant nor had a young infant were described as non-pregnant.
A second spot urine sample was collected opportunistically at a later date from a subset of participants living in the larger communities, as part of an additional study investigating hepatitis B immunity.16 This allowed us to examine the impact of correcting for within-person variation on the estimated range of UIC in the population.
Urine samples were kept cool, decanted within 2 hours and frozen. They were transported to Darwin at − 60 ° C, stored at − 80 ° C, and subsequently transported on dry ice to Westmead Hospital, Sydney, in two batches. The Westmead laboratory participates in the Ensuring the Quality of Urinary Iodine Procedures quality assurance program (Centers for Disease Control and Prevention, Atlanta, Ga, USA) and is an Asia–Pacific regional reference laboratory. Urinary iodine measurements were performed by ammonium persulfate digestion17 before a Sandell–Kolthoff reaction in a microtitre plate format.18
Standard criteria for defining population iodine status were applied to boys and non-pregnant girls — ie, a population has mild iodine deficiency if its MUIC falls in the range 50–99 μg/L and moderate deficiency if its MUIC falls in the range 20–49 μg/L.9
For pregnant and breastfeeding women in the population, MUICs < 150 μg/L and < 100 μg/L, respectively, define insufficient iodine status.9 In our study, these criteria were applied to the subgroups of pregnant girls and girls with infants under 6 months of age, respectively.
Our analysis was restricted to participants living within the DHR. Residents of the region were divided into “remote” residents (living in a community with an Aboriginal council) and “urban and other” residents, which included people living in the Darwin–Palmerston area and a small number of participants living in rural towns. Results were calculated for the DHR as a whole and for the two subareas. The 2006 Census data for the Darwin and Jabiru Indigenous Geographical Classification areas (which overlap the DHR) show that 46% of Indigenous people live in remote communities and the remainder live in Darwin–Palmerston and surrounding towns.19 We used this information, and the assumption of an equal sex ratio, to estimate a weighted MUIC value for the region.
For analysis purposes, 19 participants with a UIC below the limit of detection (10 μg/L) were assigned a value of 5 μg/L. Regression using the natural logarithm of UIC was performed to test for differences by sex, reproductive status and area of residence (urban or remote).
As there was a significant location–sex interaction, and because only one repeat specimen was collected in an urban area, we used the 82 repeat specimens collected in remote communities to estimate a corrected UIC distribution among remote-dwelling participants only. After taking the natural logarithm, repeated measures analysis of variance was performed on data from the 82 participants with repeat values to determine the between-person (sb) and total (sobs) standard deviations. A corrected UIC value was calculated for each person in the remote population by adjusting the transformed value for each person, according to the following formula:13,14
After exponentiation, the distribution of UICs for remote-dwelling participants was recalculated using the adjusted values. Statistical analyses were carried out using Stata software, version 10.1 (Stata Corp, College Station, Tex, USA).
Of the 686 participants recruited at birth, 469 were seen at follow-up and 27 had died since birth (a 71.2% participation rate among living cohort members). A further 122 participants were traced but not seen — 11 of these refused to participate and the other 111 were not interviewed for logistical reasons. Sixty-eight participants could not be traced.13
We analysed results for the DHR residents only (n = 376), rather than all cohort participants, because this is more likely to give results that are generalisable to the Aboriginal population of the DHR. All DHR residents seen provided a urine specimen; mean age was 17.8 years (range, 16–20 years). Median height and weight were 172.5 cm and 58.0 kg in the boys and 161.6 cm and 51.2 kg in the girls, respectively.
Boys and girls in both areas of residence (urban/other and remote) were iodine-deficient based on the standard criteria (Box 1). In urban areas, boys had a higher MUIC than non-pregnant girls, whereas the reverse was true in remote areas. Notably, the MUIC for urban boys was 20–30 μg/L higher than that for remote-dwelling boys or non-pregnant girls in either location (P [interaction] < 0.001). MUIC varied by only 8 μg/L between remote-dwelling boys and remote-dwelling non-pregnant girls, but this straddled the cut-off point between mild and moderate deficiency. The boys were therefore classified as having poorer iodine status than the girls. The MUIC for remote-dwelling girls who were pregnant or had young infants was lower than that for non-pregnant remote-dwelling girls (P = 0.4). When weighted to the 2006 Census, the MUIC for the DHR was 58 μg/L, compared with 54 μg/L for our study population.
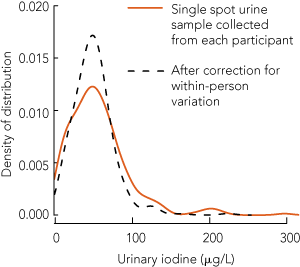
Among the 82 remote-dwelling participants (32 boys) who provided a second urine sample, the MUICs for the first and second readings were 50 μg/L and 58 μg/L, respectively (P = 0.1). The urine samples for the whole group were collected between January 2006 and November 2007 and the 82 repeat samples were collected between July and December 2007. The median number of days between the repeats was 354 (interquartile range [IQR], 196–501 days), and there was no association between the time between sample collections and the magnitude of their difference. The correlation coefficient between the two sets of log-transformed UIC readings was 0.32 (P < 0.004). The sb ÷ sobs ratio was 0.69 for the log-transformed values, which indicates that correction for within-person variation reduces the standard deviation of the log-transformed distribution by 69%. UIC distributions for all participants living in remote communities before and after correcting for within-person variation are shown in Box 2 and Box 3. Because the distribution was about log-normal, the contraction in the UIC distribution was more pronounced for high values.
Our study population of Indigenous teenagers in the DHR was iodine-deficient, like other populations in south-eastern Australia but in contrast with those in Queensland and WA.6 Urban boys had better iodine status than urban girls, whose status was similar to that of remote-dwelling boys and girls. As urban boys were the tallest and heaviest group, we hypothesise that their overall food intake, and therefore intake of all nutrients, was greater; however, we have no dietary information to enable us to assess this directly.
The MUICs in our study were below those reported elsewhere in Australia.6 Our participants were Indigenous teenagers, mostly living in remote Aboriginal communities in the tropics, whereas the other surveys examined younger children living in cities and towns, predominantly in temperate areas.6,20 This limits direct comparison between our results and those of other surveys.
There was no variation in MUIC by reproductive status among the girls; however, changes in glomerular filtration rate during pregnancy make it difficult to assess iodine excretion accurately during this lifestage. Although there have been previous iodine studies of Australian pregnant women,2-5,21,22 our study appears to be the first to assess urinary iodine in pregnant and non-pregnant females sampled from the same underlying population. Reports from other countries that have conducted iodine studies, except Switzerland, indicate that MUIC is similar in pregnant and non-pregnant women, regardless of whether overall status is in the adequate23 or deficient range.24 Our results agree with these general findings, although the small number of pregnant girls in our study should be taken into consideration. Because the reference range for MUIC is higher in pregnant women than in non-pregnant women, similar MUICs in these groups indicate a relatively greater iodine inadequacy in pregnant women.
Our study is the first documentation of iodine status in the north of the Australian continent or in a defined Aboriginal population sample. A strength of our study was the high follow-up rate, despite the difficult and remote situations, mobility of the population and cultural factors. The larger communities had to be visited on more than one occasion to find all the participants, and follow-up occurred over a 2-year period. Therefore, the low iodine concentrations found cannot be attributed to short-term aberrations in the food supply. The similarity in UICs in the first and second urine samples for participants providing two samples also indicates that the low values were not due to unusual food availability immediately before the main survey.
Ideally, we would have selected a random sample for the repeat analysis to estimate the correction factors, rather than our pragmatic convenience sample. Our results support earlier studies discussing the impact of within-person variation on the observed UIC distributions,12 and our ratios were similar to, or lower than, those observed in previous studies of other urinary electrolytes10 and vitamin and mineral intakes.25 Our study population had low overall iodine intake. It is possible that within-person variation for iodine intake or excretion might be greater in populations with more varied diets. If so, this would result in a greater correction of the distribution than we found.
The overdispersed distribution of UIC based on a single urine sample from each individual has implications for interpreting future UIC surveys in Australia, owing to the geographical variation in iodine status in populations across the continent. As an illustration, the impact of correcting for within-person variation can be estimated for the NINS6 if the log-scale 0.69 correction factor we found is assumed to apply to the general population. In Victoria, the MUIC in the NINS was 73.5 μg/L (IQR, 53–104 μg/L).6 The IQR contracts to 58–93 μg/L after correction. This shows that the proportion of the population with long-term low (< 100 μg/L) iodine status is underestimated if the population is deficient and a survey collects only one sample from each participant. In Queensland, the MUIC was 136.5 μg/L (IQR, 104–184 μg/L;6 or 112–166 μg/L after correction). This illustrates that the proportions of a population with low and high long-term iodine status are overestimated if the population has an overall adequate status and one sample is collected from each survey participant.
In Australia, after mandatory replacement of non-iodised salt with iodised salt in bread from October 2009,7 average population intakes were expected to increase by 40–50 μg/day. Post-fortification surveys collecting a single urine sample from each participant might lead to incorrect conclusions about the proportion of the population with continuing low iodine status in some areas or even excessive intakes in other areas. Our analysis indicates that if estimating the proportion with high or low status is a goal in a survey, then the survey design should include an assessment of within-person variation from all relevant subgroups.
In summary, this first study of iodine status in the NT Indigenous population found deficiency in teenagers, including those who were pregnant or had young children, before the introduction of mandatory iodine fortification in October 2009. Because bread is widely eaten,26 iodine status is expected to have improved, and we will reassess UICs in the next follow-up of our cohort. Our results are a reminder that iodine deficiency affects males and females of all ages; although most surveys target only primary school-aged children and pregnant women. We recommend that future health studies in Indigenous populations should assess the possibility that iodine deficiency may be a significant contributor to ill health and disability.
1 Urinary iodine concentrations in the Aboriginal Birth Cohort Study, 2005–2008
|
|
|
Urinary iodine concentration (μg/L) |
|
|||||||||||
Location |
Group |
Number |
Median |
IQR |
< 50 μg/L (%) |
Population iodine status* |
|||||||||
All |
All (weighted†) |
376 |
58 |
39–80 |
37.1 |
na |
|||||||||
|
All (study population†) |
376 |
54 |
34–77 |
43.6 |
na |
|||||||||
|
Boys |
183 |
55 |
29–78 |
43.7 |
Mild deficiency |
|||||||||
|
All girls |
193 |
53 |
36–76 |
43.5 |
na |
|||||||||
|
Non-pregnant girls |
158 |
55 |
36–78 |
41.1 |
Mild deficiency |
|||||||||
|
Pregnant girls |
24 |
49 |
40–72 |
50.0 |
Insufficient |
|||||||||
|
Girls with infant < 6 months of age |
11 |
39 |
31–56 |
63.6 |
Insufficient |
|||||||||
Urban and other‡ |
All (study population) |
81 |
70 |
47–91 |
25.9 |
na |
|||||||||
|
Boys§ |
45 |
77 |
61–100 |
12.8 |
Mild deficiency |
|||||||||
|
All girls |
36 |
55 |
40–79 |
38.9 |
na |
|||||||||
|
Non-pregnant girls§,¶ |
29 |
55 |
39–79 |
37.9 |
Mild deficiency |
|||||||||
Remote** |
All (study population) |
295 |
51 |
29–72 |
48.5 |
na |
|||||||||
|
Boys§ |
138 |
47 |
23–70 |
52.9 |
Moderate deficiency |
|||||||||
|
All girls |
157 |
53 |
35–74 |
44.6 |
na |
|||||||||
|
Non-pregnant girls§,†† |
129 |
55 |
35–76 |
41.9 |
Mild deficiency |
|||||||||
|
Pregnant girls†† |
18 |
47 |
36–67 |
— |
Insufficient |
|||||||||
|
Girls with infant < 6 months of age†† |
10 |
41 |
31–56 |
— |
Insufficient |
|||||||||
IQR = interquartile range. na = no criteria defined for a mixed group with varying reproductive status. * Criteria for iodine status in a general population: mild deficiency if median urinary iodine concentration (MUIC) = 50–<100 μg/L; moderate deficiency if MUIC = 20–<50 μg/L; pregnant women: insufficient status if MUIC < 150 μg/L; lactating women: insufficient status if MUIC < 100 μg/L.7 † Weighted to the 2006 Census Indigenous population;19 study population result shows the unweighted results. ‡ Darwin–Palmerston area and rural towns. § P < 0.001 for interaction between sex (boys and non-pregnant girls) and location. ¶ Data for one pregnant girl and six girls with infants < 6 months of age not shown separately. ** Community with an Aboriginal council. †† P = 0.4 for difference by reproductive status among remote-dwelling girls. |
|||||||||||||||
2 Urinary iodine concentration distribution, with and without correction for within-person variation, for Aboriginal teenagers living in remote Top End communities in the Northern Territory (n = 295)
Centile |
Raw distribution from single random sample (μg/L) |
Distribution corrected for within-person variation (μg/L) |
|||||||||||||
5th |
Below limit of detection |
Below limit of detection |
|||||||||||||
10th |
13 |
20 |
|||||||||||||
25th |
29 |
32 |
|||||||||||||
50th |
51 |
48 |
|||||||||||||
75th |
72 |
61 |
|||||||||||||
90th |
101 |
77 |
|||||||||||||
95th |
129 |
92 |
|||||||||||||
Maximum |
470 |
234 |
|||||||||||||
Proportion < 20 μg/L |
16% |
11% |
|||||||||||||
Proportion ≥ 100 μg/L |
10% |
3% |
|||||||||||||
- Dorothy E M Mackerras1,2
- Gurmeet R Singh2
- Creswell J Eastman3
- 1 Food Standards Australia New Zealand, Canberra, ACT.
- 2 Menzies School of Health Research, Institute of Advanced Studies, Charles Darwin University, Darwin, NT.
- 3 International Council for Control of Iodine Deficiency Disorders, Sydney Medical School, University of Sydney, Sydney, NSW.
We are grateful to the Aboriginal mothers and their children who agreed to be part of our study. We thank Gary Ma (Institute of Clinical Pathology and Medical Research, Westmead Hospital, Sydney, NSW) for performing the urinary iodine measurements, and Joseph McDonnell of Menzies School of Health Research, Darwin, NT for statistical advice. Gurmeet Singh collected field data and samples with the support of a National Health and Medical Research Council Program Grant.
We received a grant from the Channel 7 Foundation of South Australia to analyse iodine in our study samples. Creswell Eastman has received payment for travel and other expenses to attend board meetings of the International Council for the Control of Iodine Deficiency Disorders.
- 1. Li M, Ma G, Guttikonda K, et al. Re-emergence of iodine deficiency in Australia. Asia Pac J Clin Nutr 2001; 10: 200-203.
- 2. Chan SS, Hams G, Wiley V, et al. Postpartum maternal iodine status and the relationship to neonatal thyroid function. Thyroid 2003; 13: 873-876.
- 3. Gunton JE, Hams G, Fiegert M, McElduff A. Iodine deficiency in ambulatory participants at a Sydney teaching hospital: is Australia truly iodine replete? Med J Aust 1999; 171: 467-470. <MJA full text>
- 4. Hamrosi MA, Wallace EM, Riley MD. Iodine status in pregnant women living in Melbourne differs by ethnic group. Asia Pac J Clin Nutr 2005; 14: 27-31.
- 5. Travers CA, Guttikonda K, Norton CA, et al. Iodine status in pregnant women and their newborns: are our babies at risk of iodine deficiency? Med J Aust 2006; 184: 617-620. <MJA full text>
- 6. Li M, Eastman CJ, Waite KV, et al. Are Australian children iodine deficient? Results of the Australian National Iodine Nutrition Study. Med J Aust 2006; 184: 165-169. Correction in: Med J Aust 2008; 188: 674. <eMJA full text> <MJA full text>
- 7. Food Standards Australia New Zealand. Proposal P1003 — mandatory iodine fortification for Australia approval report. 6 Aug 2008. http://www.foodstandards.gov.au/_srcfiles/AppR_P1003_Mandatory_Iodine_Fortification_Aust%20AppR.pdf (accessed Sep 2010).
- 8. National Health and Medical Research Council. Iodine supplementation for pregnant and breastfeeding women. NHMRC public statement Jan 2010. Canberra: NHMRC, 2010. http://www.nhmrc.gov.au/_files_nhmrc/file/publications/synopses/new45_statement.pdf (accessed Sep 2010).
- 9. World Health Organization, UNICEF, International Council for the Control of Iodine Deficiency Disorders. Assessment of iodine deficiency disorders and monitoring their elimination. A guide for programme managers. 3rd ed. Geneva: WHO, 2007.
- 10. Dyer AR, Shipley M, Elliott P. Urinary electrolyte excretion in 24 hours and blood pressure in the INTERSALT Study. I. Estimates of reliability. The INTERSALT Cooperative Research Group. Am J Epidemiol 1994; 139: 927-939.
- 11. Armstrong BK, White E, Saracci R. Principles of exposure measurement in epidemiology. Oxford: Oxford University Press, 1992.
- 12. Anderson S, Karmisholt J, Pedersen KM, Laurberg P. Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br J Nutr 2008; 99: 813-818.
- 13. Subcommittee on Interpretation and Uses of Dietary Reference Intakes and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes: applications in dietary planning. Washington, DC: National Academies Press, 2003.
- 14. Australian Bureau of Statistics. Technical paper on the National Nutrition Survey: confidentialised unit record file. Canberra: ABS, 1998.
- 15. Sayers SM, Mackerras D, Singh G, et al. An Australian Aboriginal birth cohort: a unique resource for a life course study of an Indigenous population. A study protocol. BMC Int Health Hum Rights 2003; 3: 1.
- 16. Sayers S, Singh G, Mackerras D, et al. Australian Aboriginal Birth Cohort study: follow-up processes at 20 years. BMC Int Health Hum Rights 2009; 9: 23.
- 17. Pino S, Fang, S, Braverman LE. Ammonium persulphate: a safe alternative oxidising reagent for measuring urinary iodine. Clin Chem 1996; 42: 239-243.
- 18. Ohashi T, Yamaki M, Pandav C, et al. Simple microplate method for determination of urinary iodine. Clin Chem 2000; 46: 529-536.
- 19. Australian Bureau of Statistics. Population distribution, Aboriginal and Torres Strait Islander Australians, 2006. http://www.ausstats.abs.gov. au/ausstats/subscriber.nsf/0/377284127F 903297CA25733700241AC0/$File/47050_2006.pdf (accessed Dec 2010).
- 20. Seal JA, Doyle Z, Burgess JR, et al. Iodine status of Tasmanians following voluntary fortification of bread with iodine. Med J Aust 2007; 186: 69-71. <MJA full text>
- 21. Burgess JR, Seal JA, Stilwell GM, et al. A case for universal salt iodisation to correct iodine deficiency in pregnancy: another salutary lesson from Tasmania. Med J Aust 2007; 186: 574-576. <MJA full text>
- 22. Charlton KE, Gemming L, Yeatman H, Ma G. Suboptimal iodine status of Australian pregnant women reflects poor knowledge and practices related to iodine nutrition. Nutrition 2010; 26: 963-968.
- 23. Soldin OP, Soldin SJ, Pezzullo JC. Urinary iodine percentile ranges in the United States. Clin Chim Acta 2003; 328: 185-190.
- 24. Zimmermann MB. The impact of iodised salt or iodine supplements on iodine status during pregnancy, lactation and infancy. Public Health Nutr 2007; 10: 1584-1595.
- 25. Nelson M, Black AE, Morris JA, Cole TJ. Between- and within-subject variation in nutrient intake from infancy to old age: estimating the number of days required to rank dietary intakes with desired precision. Am J Clin Nutr 1989; 50: 155-167.
- 26. Brimblecombe JK, O’Dea K. The role of energy cost in food choices for an Aboriginal population in northern Australia. Med J Aust 2009; 190: 549-551. <MJA full text>





Abstract
Objective: To determine the iodine status of participants in the Aboriginal Birth Cohort Study who resided in the Darwin Health Region (DHR) in the “Top End” of the Northern Territory prior to the introduction of mandatory iodine fortification of bread.
Design, setting and participants: Participants in our study had been recruited at birth and were followed up at a mean age of 17.8 years. Spot urine samples were collected and assessed for iodine concentration at a reference laboratory. The median urinary iodine concentration (MUIC) of residents of the DHR was calculated and compared with international criteria for iodine status. Analyses were conducted for subgroups living in urban areas (Darwin–Palmerston) and remote communities (rural with an Aboriginal council). We collected a repeat sample in a subset of participants to explore the impact of within-person variation on the results.
Main outcome measure: MUIC for residents of the DHR.
Results: Urine specimens were provided by 376 participants in the DHR. Overall MUIC was 58 μg/L when weighted to the 2006 Census population. Urban boys had higher values (MUIC = 77 μg/L) than urban and remote-dwelling non-pregnant girls (MUIC = 55 μg/L), but all these groups were classified as mildly iodine deficient. Remote-dwelling boys had the lowest MUIC (47 μg/L, moderate deficiency). Pregnant girls and those with infants aged less than 6 months also had insufficient iodine status. Correction for within-person variation reduced the spread of the population distribution.
Conclusions: Previously, iodine deficiency was thought to occur only in the south-eastern states of Australia. This is the first report of iodine deficiency occurring in residents of the NT. It is also the first study of iodine status in a defined Indigenous population. Future follow-up will reassess iodine status in this group after the introduction of iodine fortification of bread.