Human infection with Rickettsia felis has been reported in most parts of the world, and R. felis has recently been confirmed in cat fleas in Western Australia. The clinical presentations of R. typhi and R. felis are similar, and in the past, the incidence of R. felis infection may have been underestimated. We describe the first reported cases of probable human R. felis infection in Australia. Two adults and three children in Victoria contracted a rickettsial disease after exposure to fleas from kittens. Molecular testing of fleas demonstrated the presence of R. felis but not R. typhi.
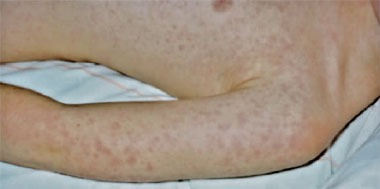
Patient B, a previously well 9-year-old girl, was admitted to a children’s hospital in Melbourne, Victoria, in April 2009 with severe abdominal pain, fevers to 39°C and a non-pruritic erythematous macular rash, initially present on the trunk and then spreading to the upper limbs and face (Box 1). The patient described a prodrome of 5 days of fever and malaise, with occasional vomiting and diarrhoea. She had been appropriately vaccinated, had no drug allergies, and did not regularly take any medication.
Serological testing was performed using indirect microimmunofluorescence assay (IFA).1,2
Initial serological analysis (in April 2009) for the presence of both spotted-fever-group and typhus-group rickettsial antibodies was undertaken on Patients B and C. The results showed the presence of typhus-group but not spotted-fever-group rickettsial antibodies. A month later (May 2009), serological testing was repeated for Patients B and C, and initial testing was done for Patients D, E and A. The tests showed rising typhus-group rickettsial antibody titres in patients B, C and E and high titres in patients A and D. In addition, Patient C showed clear evidence of seroconversion (Box 2), while both parents were negative for rickettsial antibodies. Serological testing undertaken on Cat 1 also showed the presence of typhus-group rickettsial antibodies (Box 2).
DNA was extracted from the serum of Patient C (buffy coat [white cell layer] was not available), and Cat 1, and from pooled and crushed cat fleas that were collected from cats in the group that Cat 1 and Cat 2 had come from. A rickettsial real-time polymerase chain reaction (PCR) test was performed on the extracted DNA samples.3 The fleas, but not the patient’s or cat’s serum, were positive for rickettsial DNA.
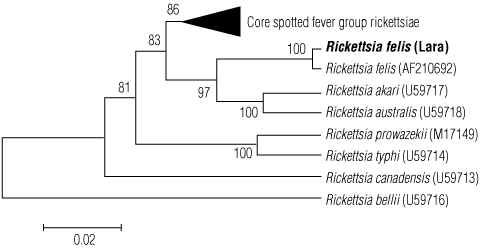
A 1077 base-pair fragment of the rickettsial citrate synthase gene was amplified and sequenced.4 This sequence was compared with the validated rickettsial species5 and showed closest phylogenetic similarity to Rickettsia felis, with a sequence similarity of 99.7% (1074/1077 base pairs). Rickettsia typhi DNA was not detected in the cat fleas. The citrate synthase gene (gltA) sequence analysis using the neighbour-joining algorithm is shown in Box 3.
The five patients described here are the first reported cases of probable human R. felis infection in Australia, and the analyses provide the first molecular evidence of R. felis in cat fleas in Victoria. It has been previously detected in cat and dog fleas in Western Australia by molecular analysis.6 Human infection with R. felis has been reported in most other parts of the world.7-10
While genetically a member of the spotted-fever rickettsia group, R. felis behaves clinically and serologically like a typhus-group rickettsia and is transmitted by fleas. Antibodies induced by R. felis react with typhus-group rickettsiae in serological tests, rather than with spotted-fever-group rickettsiae. A petechial rash is an infrequent sign of infection, and a macular or maculopapular rash is present in only 50% of patients (Box 1). The high attack rate and severity of infection noted in this cluster may be due to the heavy flea infestation that was reported.
Resolution without therapy is well described in rickettsial infection. Only two patients (B and C) received antimicrobial therapy with known activity against rickettsial species. The five patients showed a strong positive result for the presence of typhus-group antibodies. Patient C’s clear seroconversion was consistent with recent acute R. felis or R. typhi infection.7 While exposure to either R. felis or R. typhi could have led to Cat 1 producing typhus-group antibodies, only R. felis DNA was detected in the cat fleas. It is common for blood from cats infected with R. felis to be negative for rickettsial DNA,8 as in this case. Cat 1 still had antibodies to R. felis but either had cleared the infection, or the organism was present in tissues other than peripheral blood. In a previous experimental exposure of cats to R. felis-positive fleas, 13 of 16 cats were positive by serological testing using IFA, but only five of the 16 were positive by PCR.11
The human cases reported in this study were only identified serologically, and as the clinical presentations of R. typhi and R. felis are similar, R. typhi cannot be completely ruled out as the causative agent. However, given the molecular data from the cat fleas, R. felis is the more likely causative agent. In the past, the incidence of R. felis infection in patients with raised typhus group antibody levels may have been underestimated, with the causative agent probably reported as R. typhi when it may have been R. felis — a confusion that has been seen in other studies.8,9
- 1. Graves S, Stenos J, Unsworth N, Nguyen C. Laboratory diagnosis of rickettsial infection. Aust J Med Sci 2006; 27: 39-44.
- 2. Izzard L, Cox E, Stenos J, et al. Serological prevalence study of exposure of cats and dogs in Launceston, Tasmania, Australia to spotted fever group rickettsiae. Aust Vet J 2010; 88: 29-31.
- 3. Stenos J, Graves SR, Unsworth NB. A highly sensitive and specific real-time PCR assay for the detection of spotted fever and typhus group Rickettsiae. Am J Trop Med Hyg 2005; 73: 1083-1085.
- 4. Izzard L, Graves S, Cox E, et al. Novel rickettsia in ticks, Tasmania, Australia. Emerg Infect Dis 2009; 15: 1654-1656.
- 5. Fournier PE, Dumler JS, Greub G, et al. Gene sequence-based criteria for identification of new rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J Clin Microbiol 2003; 41: 5456-5465.
- 6. Schloderer D, Owen H, Clark P, et al. Rickettsia felis in fleas, Western Australia. Emerg Infect Dis 2006; 12: 841-843.
- 7. Schriefer ME, Sacci JB Jr, Dumler JS, et al. Identification of a novel rickettsial infection in a patient diagnosed with murine typhus. J Clin Microbiol 1994; 32: 949-954.
- 8. Eremeeva ME, Warashina WR, Sturgeon MM, et al. Rickettsia typhi and R. felis in rat fleas (Xenopsylla cheopis), Oahu, Hawaii. Emerg Infect Dis 2008; 14: 1613-1615.
- 9. Bitam I, Parola P, De La Cruz KD, et al. First molecular detection of Rickettsia felis in fleas from Algeria. Am J Trop Med Hyg 2006; 74: 532-535.
- 10. Pereq-Osorio CE, Zavala-Velazquez JE, Leon JJ, Zavala-Castro JE. Rickettsia felis as emergent global threat for humans. Emerg Infect Dis 2008; 14: 1019-1023.
- 11. Wedincamp J Jr, Foil LD. Infection and seroconversion of cats exposed to cat fleas (Ctenocephalides felis Bouché) infected with Rickettsia felis. J Vector Ecol 2000; 25: 123-126.





We thank Dr Aminul Islam for attempting to isolate R. felis in tissue culture.
None identified.