The majority of patients presenting to Australian emergency departments with suspected snakebite do not develop envenoming.1-3 The accepted policy in Australia is for patients to be observed and have serial blood samples tested for up to 24 hours after a bite, as well as removal of any first aid such as pressure bandages with immobilisation (PBI). This practice has some support from anecdotal case reports of delayed envenoming, but has never been formally tested in snakebite cases.4-8 According to one study from southern Queensland, some hospitals discharge asymptomatic patients with normal blood test results 4 to 6 hours after the bite. However, the study included only 34 envenomed patients, and there are concerns about its applicability to other geographical regions.9
This was a cohort study of patients with confirmed or suspected snakebite recruited to the Australian Snakebite Project (ASP). ASP recruitment and data collection processes have previously been described in detail.10-12 In brief, ASP is a national, prospective, multicentre study that recruits adults and children (aged > 2 years) with suspected or definite snakebite from over 100 hospitals. All patients have demographic and clinical information, laboratory test results and treatments recorded on a clinical research form, which is then entered into a purpose-built relational database. Ethics approval has been obtained from 19 human research ethics committees covering all institutions involved in the study.
Severe envenoming was defined as any of the following:
Venom-induced consumption coagulopathy (VICC): evidence of a complete consumption coagulopathy, indicated by either an undetectable fibrinogen level or a raised D-dimer level (at least 10 times the assay cut-off or > 2.5 mg/L), with an international normalised ratio (INR) > 3.0.
Myotoxicity: a creatine kinase (CK) level > 1000 U/L, with myalgia and/or muscle tenderness.
Neurotoxicity: with either two nerve groups (eg, ocular and bulbar) involved, respiratory muscle paralysis, or requirement for intubation or mechanical ventilation.
Thrombotic microangiopathy: defined as the presence of intravascular haemolysis on the blood film, thrombocytopenia and an abnormal creatinine level with or without acute renal failure.13
Based on biological plausibility and visual inspection of early serial laboratory test results comparing patients with severe or minor envenoming to non-envenomed patients, only early changes in coagulation test results, CK level, white cell count (WCC) and lymphocyte count were further analysed for diagnostic utility. The cut-off values used to define abnormal results were: INR > 1.2, aPTT outside the reference range (depending on the individual laboratory), CK > 250 U/L, and WCC > 11.0 × 109/L. Based on previous concerns about cases of isolated neurotoxicity occurring in death adder envenoming,9 we included a neurological exam-ination in cases of neurotoxicity as part of the analysis.
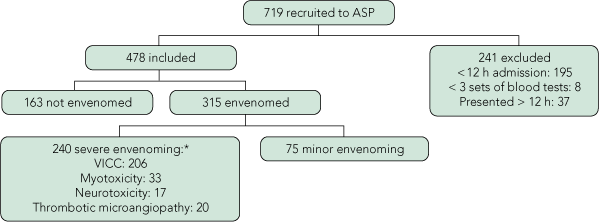
There were 478 patients in three groups: 240 had severe envenoming, 75 had minor envenoming and 163 were not envenomed (Box 1, Box 2). PBI were used on 370 patients (77%) at some time.
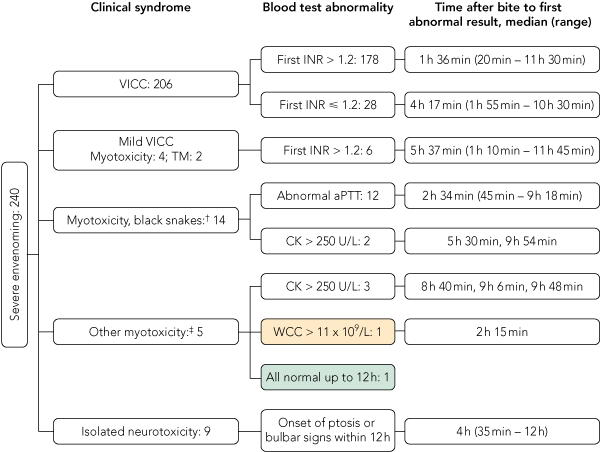
In 178 of the 206 patients (86%), the INR was abnormal (> 1.2) on the first set of tests using blood samples taken a median of 1 hour and 36 minutes after the bite (range, 20 min – 11 h 30 min) (Box 3). The remaining 28 patients had a normal INR recorded before VICC developed (including one patient who had a second normal INR at 3 hours and 45 minutes after the bite). For these 28 patients, the median time after the bite to the normal INR was 1 hour (range, 7 min – 3 h 45 min) and the median time after the bite to the first abnormal INR was 4 hours and 17 minutes (range, 1 h 55 min – 10 h 30 min). In seven patients where the time from the bite to the first abnormal INR was greater than 6 hours, this reflected a delay of 4 or more hours between the first (normal) INR and the second (abnormal) INR. For all patients with VICC, the median time to an abnormal INR was 1.8 hours (range, 20 min – 11 h 30 min) (Box 4, A).
Two patients with myotoxicity did not have an abnormal CK level or aPTT within 12 hours (Box 3). One of these patients had a normal CK level but an elevated WCC at 2 hours and 15 minutes, and the next sample was not taken until 33 hours and 30 minutes after the bite, which then had a CK level of 4770 U/L. The second patient had a CK level of 220 U/L at 4 hours and no further testing until 20 hours after the bite, with a CK level in that sample of 691 U/L. This patient developed myotoxicity with localised myalgia and a CK level peaking at 12 028 U/L at 46 hours after the bite, with ptosis and diplopia but no VICC.
For patients with myotoxicity, the median time to an abnormal CK level was 9.1 hours (range, 45 min – 33 h 30 min), and median time to an abnormal aPTT was 4.2 hours (range, 45 min – 9 h 30 min) (Box 4, A).
There were nine cases of isolated severe neurotoxicity, and all nine were from death adder bites. The median time of onset of neurotoxicity after the bite was 4 hours (range, 35 min – 12 h) (Box 4, A). Eight patients had ptosis, and the ninth had bulbar weakness. Three patients who developed respiratory muscle paralysis and required mechanical ventilation had evidence of neurotoxicity after 35 minutes, 3 hours, and 4 hours, respectively.
An abnormal WCC developed in 192 of the 240 patients with severe envenoming (80%), and the median time to an abnormal WCC in these patients was 5.7 hours (range, 30 min – 26 h 45 min) (Box 4, A). Leukocytosis occurred in similar numbers of mildly and severely envenomed patients, and only identified one case of severe envenoming not identified by the other three tests within 12 hours in this study (Box 3). Patients with brown snake (Pseudonaja spp) bites were less likely to have leukocytosis occur at any time, compared with patients with bites from the tiger snake group (Notechis spp and T. carinatus) or red-bellied black snakes (P. porphyriacus).
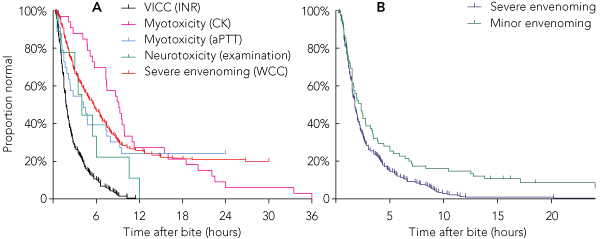
Using a combination of three laboratory tests (INR, aPTT and CK) and repeated neurological examination, 238 of the 240 patients with severe envenoming (99%) had an abnormal laboratory test result or evidence of neurotoxicity by 12 hours (Box 3 and Box 4, B). Both patients who did not have abnormal test results within 12 hours had myotoxicity and did not have laboratory tests performed at 6 or 12 hours, when it was likely their CK levels would have been elevated. There were 222 patients with severe envenoming who presented within 6 hours of the bite, and 213 of these (96%) had documented abnormal laboratory test results at 6 hours. Applying the same laboratory criteria to the minor envenoming cases, 64 of the 75 patients (85%) had abnormal results within 12 hours (Box 4, B). The median time to an abnormality (laboratory test result or neurological examination) was 1.75 hours (range, 20 min – 33 h 30 min) for severe envenoming and 2.1 hours (15 min – 30 h 45 min) for minor envenoming.
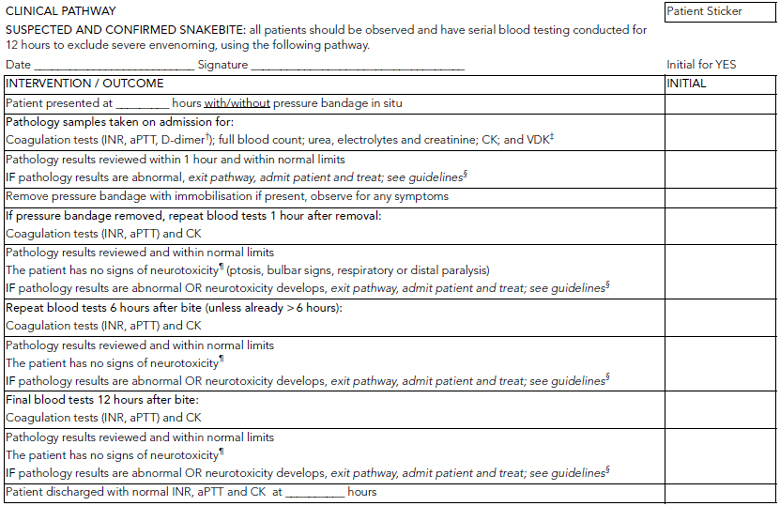
This study suggests that the combination of tests for INR, aPTT and CK level and serial neurological examinations is able to reliably detect envenomed patients within 12 hours. No single test was sufficiently useful to exclude severe envenoming within 12 hours, and a combination of the three tests, which are relevant to different envenoming syndromes, was most appropriate. The study supports an observation period of 12 hours for suspected snakebite, with repeat laboratory testing and neurological assessments performed on admission and at 6 hours and 12 hours after the bite (Box 5).
Although PBI were used for most patients and may have delayed the onset of envenoming in some, the majority had evidence of envenoming on admission despite the use of PBI. Current recommendations are that repeat blood tests should be performed 1 hour after PBI removal because envenoming can develop rapidly after removal of effective PBI.14
Most literature suggests that only 5% to 10% of snakebite patients develop severe envenoming,1,3,8,15-19 so much of the emergency department or hospital workload is the assessment of suspected snakebite to exclude severe envenoming. This study provides an approach to assessing these patients based on a large number of severely envenomed patients. Coagulation studies were the most useful early laboratory parameters (Box 4, A) because the major clinical syndrome was VICC. The aPTT was an early detector of myotoxicity because of the presence of an anticoagulant coagulopathy in black snake envenoming and of VICC in tiger snake and taipan envenoming — the snake types that most often cause myotoxicity. CK level was not a good early detector of myotoxicity and in some cases took over 12 hours to become abnormal in affected patients, although this was often due to delayed repeat CK testing (Box 4, A).
The WCC and lymphocyte count were less useful as early detectors of envenoming because, unlike VICC and myotoxicity, where the INR and CK level, respectively, must become abnormal by definition, this is not the case for WCC, which remained normal for the duration of observation in some patients with severe envenoming. In this study, an abnormal WCC only identified one further case of severe envenoming that was not detected within 12 hours by the other tests (Box 3), and it is likely that if a CK level had been tested at 12 hours in this patient, it would have been abnormal. The WCC did not reflect severity of envenoming and was more often raised in cases of red-bellied black snake envenoming, which did not commonly cause severe envenoming, compared with brown snake envenoming, which did commonly cause severe envenoming with VICC. The WCC was therefore not included with the three laboratory tests we selected to identify whether envenoming had occurred.
Another problem is that there were few cases of myotoxicity (33) and isolated neurotoxicity (9) in our study. These syndromes are a major source of delayed or missed envenoming, so larger studies of these groups are needed to confirm our approach in such cases. In addition, modification to the assessment pathway proposed in Box 5 may be needed for geographical regions with venomous snake fauna not well represented in our dataset.
Another limitation was the definition of non-envenoming, which was based on patients having no clinical effects and normal blood test results on discharge, which may have been soon after 12 hours in some cases. There is a possibility that some of these patients developed envenoming after discharge. However, Box 4, B suggests that the proportion of patients developing abnormal test results after 12 hours would be negligible. In addition, if patients re-presented to hospital with delayed onset of envenoming, this would have been identified in the ASP database by additional laboratory tests being ordered.
In conclusion, in this series of snakebite patients, the combination of INR, aPTT and CK level, together with repeated neurological assessments, detected all but two cases of severe envenoming within 12 hours. Most cases of severe envenoming (96%) were identified at 6 hours using this combination of tests. The results of this study can be used to develop potential clinical pathways, such as that shown in Box 5. However, such protocols need to be tested in a prospective study of patients with suspected snakebite.
3 Application of three laboratory tests (INR, aPTT and CK) and a neurological examination, and identification of patients with severe envenoming by abnormal test results within 12 hours of snakebite*
 | |||||||||||||||
4 Plots of the proportion of envenomed patients with normal laboratory test results versus time after bite
 | |||||||||||||||
- Graham Ireland1
- Simon G A Brown2
- Nicholas A Buckley3
- Jeff Stormer4
- Bart J Currie5
- Julian White6
- David Spain1
- Geoffrey K Isbister4,5,7
- 1 Emergency Department, Gold Coast Hospital, Gold Coast, QLD.
- 2 Centre for Clinical Research in Emergency Medicine, Western Australian Institute for Medical Research and Emergency Medicine, Royal Perth Hospital, University of Western Australia, Perth, WA.
- 3 Prince of Wales Hospital Medical School, University of New South Wales, Sydney, NSW.
- 4 Department of Clinical Toxicology and Pharmacology, Calvary Mater Newcastle Hospital, Newcastle, NSW.
- 5 Tropical and Emerging Infectious Diseases, Menzies School of Health Research, Charles Darwin University, Darwin, NT.
- 6 Department of Toxinology, Women’s and Children’s Hospital, Adelaide, SA.
- 7 Discipline of Clinical Pharmacology, University of Newcastle, Newcastle, NSW.
This article was written on behalf of the ASP clinical investigators who recruited patients to the study: Michael Taylor (Albury Base Hospital), Yusuf Nagree (Armadale Hospital), Fergus Kerr (Austin Hospital), Conrad Macrokanis (Broome Hospital), Garry Wilkes (Bunbury Hospital), Robert Bonnin, Richard Whitaker and Lambros Halkidis (Cairns Base Hospital), Geoff Isbister (Calvary Mater Newcastle Hospital), Nicholas Buckley (Canberra Hospital), Alan Tankel (Coffs Harbour Base Hospital), Randall Greenberg (Dubbo Base Hospital), Mark Webb (Flinders Medical Centre), Rod Ellis (Fremantle Hospital and Rockingham Hospital), David Spain and Graham Ireland (Gold Coast Hospital), Mark Miller (John Hunter Hospital), Chris Gavaghan (Lismore Base Hospital), Anna Holdgate (Liverpool Hospital), Todd Fraser (Mackay Hospital), Mark Coghlan (Nambour Hospital), Colin Page (Princess Alexandra Hospital), Peter Thompson (Rockhampton Hospital), Julian White (Royal Adelaide Hospital and Women’s and Children’s Hospital, Adelaide), Tanya Gray (Royal Children’s Hospital, Brisbane), Bart Currie (Royal Darwin Hospital), Justin Yeung, Simon Brown and David McCoubrie (Royal Perth Hospital), Mark Little and Ovidiu Pascu (Sir Charles Gairdner Hospital), Nick Ryan (Tamworth Hospital), Ben Close (Townsville Hospital), Shane Curran (Wagga Wagga Base Hospital), Naren Gunja (Westmead Hospital); and the ASP laboratory investigators including Margaret O’Leary, Sarah Just and Vaughan Williams. We also acknowledge the many referrals from poison information centres and clinical toxicologists, and help of the many other nurses, doctors and laboratory staff in recruiting patients and collecting samples. This study was supported in part by National Health and Medical Research Council (NHMRC) Project Grant 490305, and Simon Brown and Geoff Isbister are both funded by NHMRC Clinical Career Development Awards.
Julian White has received travel assistance from CSL Ltd (producer of antivenom) to attend international meetings as an invited speaker.
- 1. Isbister GK, Currie BJ. Suspected snakebite: one year prospective study of emergency department presentations. Emerg Med (Fremantle) 2003; 15: 160-169.
- 2. Jelinek GA, Breheny FX. Ten years of snake bites at Fremantle Hospital. Med J Aust 1990; 153: 658-661.
- 3. Hughes A. Observation of snakebite victims: is twelve hours still necessary? Emerg Med (Fremantle) 2003; 15: 511-517.
- 4. Gavaghan CF, Sparkes G. Delayed myotoxicity in snake envenoming by the tiger snake group. Emerg Med (Fremantle) 2003; 15: 497-499.
- 5. Hood VL, Johnson JR. Acute renal failure with myoglobinuria after tiger snake bite. Med J Aust 1975; 2: 638-641.
- 6. Furtado MA, Lester IA. Myoglobinuria following snakebite. Med J Aust 1968; 1: 674-676.
- 7. White J. Elapid envenomation. In: Covacevich J, Davie P, Pearn J, editors. Toxic plants and animals: a guide for Australia. 1st ed. Brisbane: Queensland Museum, 1987.
- 8. Mead HJ, Jelinek GA. Suspected snakebite in children: a study of 156 patients over 10 years. Med J Aust 1996; 164: 467-470. <MJA full text>
- 9. Currie BJ. Snakebite in Australia: moving from anecdotes to prospective studies. Emerg Med (Fremantle) 2003; 15: 406-408.
- 10. Isbister GK, Brown SG, MacDonald E, et al. Current use of Australian snake antivenoms and frequency of immediate-type hypersensitivity reactions and anaphylaxis. Med J Aust 2008; 188: 473-476. <MJA full text>
- 11. Isbister GK, Duffull SB, Brown SG; ASP Investigators. Failure of antivenom to improve recovery in Australian snakebite coagulopathy. QJM 2009; 102: 563-568.
- 12. Isbister GK, Williams V, Brown SG, et al. Clinically applicable laboratory end-points for treating snakebite coagulopathy. Pathology 2006; 38: 568-572.
- 13. Isbister GK, Little M, Cull G, et al. Thrombotic microangiopathy from Australian brown snake (Pseudonaja) envenoming. Intern Med J 2007; 37: 523-528.
- 14. Pearn J, Morrison J, Charles N, Muir V. First-aid for snake-bite: efficacy of a constrictive bandage with limb immobilization in the management of human envenomation. Med J Aust 1981; 2: 293-295.
- 15. Barrett R, Little M. Five years of snake envenoming in far north Queensland. Emerg Med (Fremantle) 2003; 15: 500-510.
- 16. Munro JG, Pearn JH. Snake bite in children: a five year population study from South-East Queensland. Aust Paediatr J 1978; 14: 248-253.
- 17. Jamieson R, Pearn J. An epidemiological and clinical study of snake-bites in childhood. Med J Aust 1989; 150: 698-702.
- 18. White J. Patterns of elapid envenomation and treatment in South Australia. Toxicon 1983; 21 Suppl 3: 489-491.
- 19. Jelinek GA, Hamilton T, Hirsch RL. Admissions for suspected snake bite to the Perth adult teaching hospitals, 1979 to 1988. Med J Aust 1991; 155: 761-764.





Abstract
Objectives: To determine which laboratory tests are first associated with severe envenoming after a snakebite, when (ie, how long after the bite) the test results become abnormal, and whether this can determine a safe observation period after suspected snakebite.
Design, patients and setting: Prospective cohort study of 478 patients with suspected or confirmed snakebite recruited to the Australian Snakebite Project from January 2002 to April 2009, who had at least three sets of laboratory test results and at least 12 hours of observation in hospital after the bite. Severe envenoming was defined as venom-induced consumption coagulopathy (VICC), myotoxicity, neurotoxicity or thrombotic microangiopathy.
Main outcome measures: International normalised ratio (INR), activated partial thromboplastin time (aPTT), creatine kinase (CK) level, and neurological examination.
Results: There were 240 patients with severe envenoming, 75 with minor envenoming and 163 non-envenomed patients. Of 206 patients with VICC, 178 had an INR > 1.2 (abnormal) on admission, and the remaining 28 had an INR > 1.2 within 12 hours of the bite. Of 33 patients with myotoxicity, a combination of CK > 250 U/L and an abnormal aPTT identified all but two cases by 12 hours; one of these two was identified within 12 hours by leukocytosis. Nine cases of isolated neurotoxicity had a median time of onset after the bite of 4 hours (range, 35 min – 12 h). The combination of serial INR, aPTT and CK tests and repeated neurological examination identified 213 of 222 severe envenoming cases (96%) by 6 hours and 238 of 240 (99%) by 12 hours.
Conclusion: Laboratory parameters (INR, aPTT and CK) and neurological reassessments identified nearly all severe envenoming cases within 12 hours of the bite, even in this conservative analysis that assumed normal test results if the test was not done.