Vitamin D deficiency is highly prevalent in Australia.1 Evidence of beneficial effects of vitamin D on bone health and muscle strength, and more recently on other organ systems, has emerged.2-7 Vitamin D replacement should increase serum 25-hydroxyvitamin D (25(OH)D) levels to over 50 nmol/L, and ideally to above 75 nmol/L.1,8
Vitamin D deficiency in Australia is most commonly treated with preparations containing 1000 IU of cholecalciferol. However, in moderate to severe vitamin D deficiency, cholecalciferol 3000 to 5000 IU daily for up to 3 months is usually required, potentially reducing compliance with treatment.1,9 High-dose oral formulations are available in many countries, but are not approved for routine use in Australia.10-13 The Australian experience of high-dose vitamin D therapy is limited and few studies of high-dose vitamin D have assessed urinary calcium excretion.14,15
Inpatients at the Western and Sunshine Hospitals in Melbourne with serum levels of 25(OH)D = 50 nmol/L, between March 2007 and January 2008, were eligible. Patients receiving vitamin D supplements or with hypercalcaemia, primary hyperparathyroidism, Paget disease of bone, active malignancy, thyrotoxicosis, hypercalciuria, serum creatinine concentrations of > 150 µmol/L, or a history of nephrolithiasis were excluded. Ethical approval was obtained from the Melbourne Health Human Research Ethics Committee. The study was registered with the Australian Clinical Trials Registry (ACTRN 12607000338460).
Patients were randomly assigned to either a high-dose group or a low-dose group. Each patient in the high-dose group received an oral tablet of cholecalciferol 50 000 IU (Cal-D-Forte, API Consumer Brands) daily for 10 days (a total dose of cholecalciferol 500 000 IU). Each patient in the low-dose group received three tablets of cholecalciferol 1000 IU (OsteVit-D, Key Pharmaceuticals) daily for 30 days, followed by one tablet daily for 60 days (a total dose of 150 000 IU). All patients were prescribed calcium citrate 500 mg daily. Compliance with cholecalciferol treatment was assessed by direct questioning (= 80% of tablets taken).
The primary end points were the absolute change in serum 25(OH)D and the achievement of serum 25(OH)D > 50 nmol/L at 3 ± 1 months. The half-life of 25(OH)D in serum is in the range of 22–28 days. Thus, the period of this study is equivalent to 3–4 half-lives, a minimum period with a high probability of measuring a steady-state level. For safety, patients were monitored for the development of hypercalciuria (urine calcium > 7.5 mmol/day) at 3 months and hypercalcaemia (corrected calcium > 2.6 mmol/L) at 3 weeks and 3 months. Changes in serum parathyroid hormone (PTH), alkaline phosphatase (ALP) and corrected calcium were also analysed.
Serum 25(OH)D (radioimmunoassay after extraction [Diasorin, Stillwater, Minn, USA], coefficient of variation, 10.7%, 9.5% and 11.5% at levels of 33, 61 and 117 nmol/L, respectively), calcium, albumin, PTH, ALP and creatinine, as well as 24-hour urine calcium excretion, were measured at baseline and 3 ± 1 months. Serum calcium and albumin were also measured at 3 weeks. Calculated corrected calcium was used for analyses.
Analyses were performed on test results of all patients who attended their final assessment at 3 ± 1 months (SPSS version 16, SPSS Inc, Chicago, Ill, USA). Nonparametric tests were used because of the small number of participants (the Wilcoxon signed-rank test or Mann–Whitney U test for continuous variables, and chi-square test for proportions). The effect of season of recruitment on baseline and changes in serum 25(OH)D was evaluated using multivariate analysis of covariance (ANCOVA). A P value of < 0.05 was considered statistically significant.
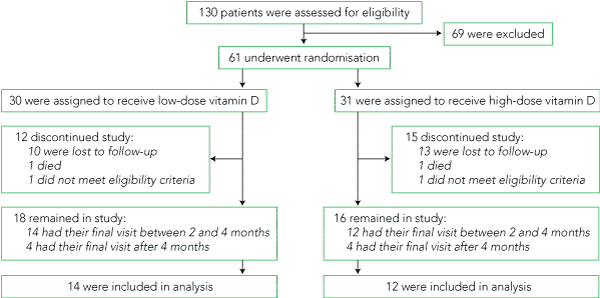
Of 130 patients assessed for eligibility, 61 met the criteria and gave written, informed consent to participate (Box 1). Two patients were retrospectively excluded for unsuspected Paget disease of bone or active malignancy. Of the remaining 59 patients, one in each group died of acute myocardial infarction, and 23 failed to re-attend. Of the 34 completers, 26 had their final assessment at 3 ± 1 months. The eight subjects who were tested 4–6 months from baseline had a significantly smaller increase in serum 25(OH)D than those who completed within 3 ± 1 months (38.7 v 52.4 nmol/L; P = 0.02), and for this reason they were excluded from the analysis. There were no statistically significant differences at baseline between completers (n = 26) and non-completers (n = 33), except that men were more likely to be non-completers (P = 0.007). Baseline characteristics of the patients who completed the study at 3 ± 1 months were similar between treatment groups (Box 2).
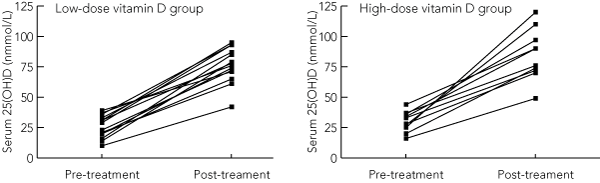
Changes in biochemical variables are shown in Box 3. Pre- and post-treatment serum 25(OH)D levels for each individual by group are depicted in Box 4. The high-dose group (12 participants) and low-dose group (14 participants) had mean ± SD baseline 25(OH)D levels of 28 ± 9 nmol/L and 26 ± 9 nmol/L, respectively. A third of patients in the high-dose group and half those in the low-dose group had severe vitamin D deficiency (serum 25(OH)D < 25 nmol/L). The mean increase in serum 25(OH)D was similar in both groups, reaching 55 nmol/L (95% CI, 40–69) and 51 nmol/L (95% CI, 44–58) in the high- and low-dose groups, respectively. Serum 25(OH)D reached a mean level of 85 ± 21 nmol/L and 77 ± 15 nmol/L for the high- and low-dose groups, respectively, at 3 months (P = 0.4 for the difference between groups). There was no effect of season on either baseline or changes in serum 25(OH)D.
In the high- and low-dose groups, 9/10 and 13/14 of patients attained serum 25(OH)D levels > 50 nmol/L, while 6/10 and 8/14 reached levels = 75 nmol/L, respectively. Post-treatment serum 25(OH)D values were all within the physiological range (< 200 nmol/L).16,17 The highest 3-month serum level of 25(OH)D (120 nmol/L) occurred within the high-dose group.
Serum calcium was normal in the 20 patients who had measurements taken 2–5 weeks after treatment initiation. At 3 months, corrected calcium increased by 0.1 mmol/L in the low-dose group (P = 0.03), but no significant change occurred in the high-dose group. No patient developed hypercalcaemia. Three patients (two in the low-dose and one in the high-dose group) had 24-hour urine calcium excretions > 7.5 mmol/day, and two patients had > 10 mmol/day. No nephrolithiasis was reported.
There was a statistically significant rise in serum creatinine in the low-dose treatment group from 78 ± 23 to 91 ± 40 mmol/L (P = 0.004). However, all 3-month serum creatinine concentrations were within the normal range, except for one patient in the low-dose group, who developed worsening renal impairment (serum creatinine increased from 140 to 220 µmol/L).
A regimen of high-dose oral cholecalciferol was as effective at increasing serum 25(OH)D concentrations at 3 months as a longer, low-dose regimen. In both groups, = 90% of patients achieved levels of 25(OH)D > 50 nmol/L, and about 60% achieved levels of = 75 nmol/L, comparable with previous studies of vitamin D supplementation.15,18 Consistent with Australian data, season did not influence baseline 25(OH)D or increases in serum 25(OH)D concentrations, implying behavioural factors are more important.19
No patient developed nephrolithiasis, but longer follow-up and radiographic studies would be required to exclude this possibility. The association between hypercalciuria with vitamin D therapy and nephrolithiasis is unclear.15 A systematic review did not find evidence of adverse effects from higher intakes of vitamin D and noted instead that postmenopausal women who developed nephrolithiasis had higher calcium intakes.20
There are limitations to our study. Firstly, neither participants nor researchers were blinded to the treatment arms, which could have resulted in cross-contamination of the treatment groups. However, this is likely to mirror clinical practice. Secondly, there was a high drop-out rate, with only 26 of the original 59 enrolled subjects (44%) attending the final assessment within the prescribed time. Although the completers and non-completers were similar, a sex difference between these groups may have influenced the results. Thirdly, compliance was assessed by questionnaire, which could be affected by recall bias. Compliance with the daily calcium supplement was not recorded, so any effect of coadministration of cholecalciferol on compliance with calcium is unknown.
It is surprising that reported compliance with the low-dose vitamin D regimen was 86%. Indeed, low adherence rates with daily cholecalciferol have been reported in previous studies.21,22 While unexpected, it is possible that the lower compliance in the high-dose group (17%) was due to patient reluctance to take what they had interpreted to be a “megadose”. Future studies would be strengthened by long-term follow-up to assess the safety and clinical relevance of the hypercalciuria, which developed in a few patients. Compliance with high-dose therapy could be optimised by patient education or by giving all ten 50 000 IU tablets at once under observation.18
This study has raised local awareness of the prevalence of hypovitaminosis D in hospitalised patients. At present, authority to prescribe tablets of cholecalciferol 50 000 IU can only be obtained in Australia through special access schemes or under section 19(5) of the Therapeutic Goods Act via hospital or community pharmacies; or via private prescriptions through compounding pharmacists at a cost of $20 for 10 tablets. The cost of the low-dose regimen is about $30 at retail pharmacies. Replacement with high-dose oral cholecalciferol may be more practical and cost-effective than longer-term, low-dose treatment. Improved access to high-dose cholecalciferol in Australia is needed.23
2 Baseline characteristics of the participants who completed the study within 3 ± 1 months (n = 26)
|
|
|||||||||||||||
|
Participants of European ancestry, n |
|||||||||||||||
|
|
|||||||||||||||
3 Mean absolute change (95% CI) in biochemical variables after 3 ± 1 months of treatment
|
Difference between groups in mean change (95% CI) |
|||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Received 18 September 2009, accepted 17 January 2010
- Kathryn L Hackman1
- Claudia Gagnon1
- Roisin K Briscoe1
- Simon Lam2,3
- Mahesan Anpalahan1
- Peter R Ebeling1
- 1 Department of Medicine, University of Melbourne, Melbourne, VIC.
- 2 Departments of General Medicine and Rheumatology, Austin Health, Melbourne, VIC.
- 3 Rheumatology Department, Western Health, Melbourne, VIC.
We would like to thank Key Pharmaceuticals, Australia, for providing low-dose cholecalciferol. We give special thanks also to the University of Melbourne for funding support.
None identified.
- 1. Working Group of the Australian and New Zealand Bone and Mineral Society, Endocrine Society of Australia and Osteoporosis Australia. Vitamin D and adult bone health in Australia and New Zealand: a position statement. Med J Aust 2005; 182: 281-285. <MJA full text>
- 2. Bischoff HA, Stahelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomised controlled trial. J Bone Miner Res 2003; 18: 343-351.
- 3. Chatfield SM, Brand C, Ebeling PR, et al. Vitamin D deficiency in general medical inpatients in summer and winter. Intern Med J 2007; 37: 377-382.
- 4. Holick M. Vitamin D deficiency [review]. N Engl J Med 2007; 357: 266-281.
- 5. Flicker L, Mead K, MacInnis RJ, et al. Serum vitamin D and falls in older women in residential care in Australia. J Am Geriatr Soc 2003; 51: 1533-1538.
- 6. Lin R, White JH. The pleiotropic actions of vitamin D. Bioessays 2004; 26: 21-28.
- 7. Neale RE, Webb PM, van der Pols JC. Vitamin D for prevention of chronic disease: the need for continued research. Intern Med J 2008; 38: 813-815.
- 8. Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 2007; 85: 649-650.
- 9. Aloia JF, Patel M, Dimaano R, et al. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr 2008; 87: 1952-1958.
- 10. Wu F, Staykova T, Horne A, et al. Efficacy of an oral, 10 day course of high dose cholecalciferol in correcting vitamin D deficiency. N Z Med J 2003; 116: U536.
- 11. Mastaglia SR, Mautalen CA, Paris MS, et al. Vitamin D2 dose required to rapidly increase 25OHD in osteoporotic women. Eur J Clin Nutr 2006; 60: 681-687.
- 12. Khaw K-T, Scragg R, Murphy S. Single dose cholecalciferol suppresses the winter increase in parathyroid hormone concentrations in healthy older men and women: A randomized trial. Am J Clin Nutr 1994; 59: 1040-1044.
- 13. Weisman Y, Schen RJ, Eisenberg Z, et al. Single oral high dose Vitamin D3 prophylaxis in the elderly. J Am Geriatr Soc 1986; 34: 515-518.
- 14. Diamond TH, Ho KW, Rohi PG, Meerkin, M. Annual intramuscular injection of a megadose of cholecalciferol for treatment of vitamin D deficiency: efficacy and safety data. Med J Aust 2005; 183: 10-12. <MJA full text>
- 15. Veith R, Chan P-C R, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr 2001; 73: 288-294.
- 16. Vieth R. Vitamin D toxicity, policy, and science. J Bone Miner Res 2007; 22 Suppl 2: 64-68.
- 17. Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 1999; 69: 842-856.
- 18. Bacon CJ, Gamble GD, Horne AM, et al. High-dose oral vitamin D3 supplementation in the elderly. Osteoporos Int 2009; 20: 1407-1415.
- 19. van der Mei IA, Ponsonby A-L, Engelsen O, et al. The high prevalence of vitamin D insufficiency across Australian populations is only partly explained by season and latitude. Environ Health Perspect 2007; 115: 1132-1139.
- 20. Cranney A, Horsley T, O’Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep) 2007; 158: 1-235.
- 21. Grant A, Avenell A, Campbell M, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomized Evaluation of Calcium Or vitamin D, RECORD): a randomized placebo-controlled trial. Lancet 2005; 365: 1621-1628.
- 22. Porthouse J, Cockayne S, King C, et al. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ 2005; 330: 1003-1008.
- 23. Ebeling, PR. Mega therapy for vitamin D deficiency [editorial]. Med J Aust 2005; 183: 4-5. <MJA full text>





Abstract
Objective: To compare the efficacy and safety of a 10-day, high-dose v a 3-month, continuous low-dose oral cholecalciferol course in a vitamin D deficient population. The primary end points were the change in serum 25-hydroxyvitamin D (25(OH)D) concentrations at 3 months and the development of hypercalcaemia and hypercalciuria.
Design, setting and participants: Fifty-nine vitamin D deficient inpatients (serum 25(OH)D ≤ 50 nmol/L) were enrolled in a prospective, randomised, open-label trial. Participants were randomly assigned to a high-dose regimen of cholecalciferol 50 000 IU daily for 10 days or a 3-month, continuous low-dose cholecalciferol regimen of 3000 IU daily for 30 days, followed by 1000 IU daily for 60 days. Both groups received calcium citrate 500 mg daily.
Results: Twenty-six patients completed the study within 3 ± 1 months. The mean increases in serum 25(OH)D were similar in both the high- and low-dose groups (to 55 v 51 nmol/L, respectively; P = 0.9). There was no significant difference in the proportion of subjects who attained serum 25(OH)D concentrations > 50 nmol/L between the high- and low-dose groups (9/10 v 13/14, respectively; P = 1.0). Hypercalciuria (urine calcium > 7.5 mmol/day) occurred in three patients (two low-dose, one high-dose), while renal impairment worsened in one patient. No patient developed hypercalcaemia (corrected calcium > 2.6 mmol/L), vitamin D toxicity (25(OH)D > 200 nmol/L) or nephrolithiasis during the study.
Conclusion: Both the 10-day, high-dose and the 3-month, low-dose cholecalciferol regimens effectively increased serum 25(OH)D to within the normal range. The high-dose regimen may be an effective and cheap alternative for patients with vitamin D deficiency.
Trial registration: Australian Clinical Trials Registry ACTRN 12607000338460.