The mumps vaccine used in Australia is a live attenuated vaccine derived from the Jeryl Lynn strain (genotype A).1 A single dose produces measurable antibodies in more than 97% of people,2,3 and vaccine effectiveness is reported as about 80% after one dose and about 88% after two doses.2
Theoretically, all residents of Western Australia born since 1981 should have received two doses of mumps vaccine, as a single dose was introduced at age 12 months in 1981, followed by a two-dose schedule in 1994 (with the second dose initially at age 12 years and later at age 4–5 years). However, vaccine coverage in the 1980s was not high, and uptake of the second dose varied.4 A catch-up program was conducted for children aged 4–16 years in 1998, but school-based delivery was limited to primary schools,4 and a booster measles–mumps–rubella (MMR) campaign for 18–30-year-olds in 2000–2005 had poor uptake in WA (unpublished data, Communicable Disease Control Directorate, WA Department of Health, Perth, WA).
Nevertheless, between 1993 and 2006, the number of notified cases of mumps in WA fluctuated between nine and 37 per year, with most cases in non-Aboriginal people. However, in 2007, notified cases rose to 109 per year, as a result of an outbreak among Aboriginal people living in the Kimberley region. This contrasted with a maximum of four cases per year notified from this region during the previous 14 years (unpublished data, Communicable Disease Control Directorate). In 2007, the Kimberley region had an estimated total population of 34 345, of whom 51% were Aboriginal.5 The region has six towns, with populations of 2000–15 000 each, and more than 200 discrete Aboriginal communities, with populations ranging from a few families to over 500 people. The mumps outbreak was epidemiologically linked to a cluster of cases in a boarding school in Darwin in the Northern Territory, which borders the Kimberley region.6
Vaccination status was confirmed using the Australian Childhood Immunisation Register7 and the WA Health Care and Related Encounters (HCARe) databases8 and was classified according to the number of doses of mumps vaccine received (one, two or unknown). Notification rates for the Kimberley region were calculated from population estimates derived from the Australian Bureau of Statistics 2006 census using the method of Codde.5 Vaccine efficacy (VE) was calculated using the screening method:
where x = proportion of cases vaccinated, and y = proportion of population vaccinated.9
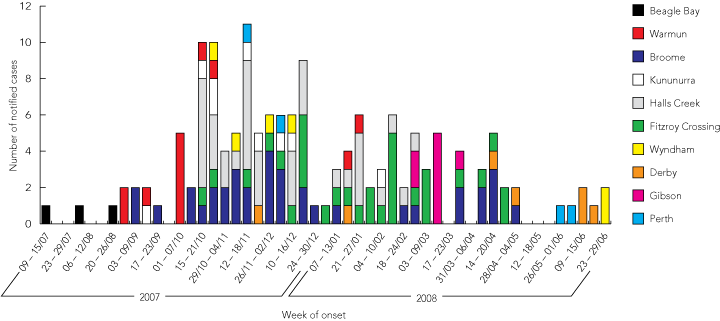
The index case was one of 12 Aboriginal students from Beagle Bay, a remote community 120 km from Broome, who returned home for the holidays from a boarding school in Darwin where a cluster of mumps cases had been identified. Two further cases occurred in Beagle Bay, followed by cases in the Kimberley community of Warmun, and the towns of Broome, Kununurra, Halls Creek, Fitzroy Crossing, Wyndham and Derby. The epidemic peaked in October–November 2007 (Box 1).
Of the outbreak cases, 92% (141/153) were in Aboriginal people, 49% of patients (75/153) were male, and the median age was 18 years (range, 2–63 years). Most patients (82%, 126/153) were aged 5–29 years, with 22% (34/153) aged 15–19 years (Box 2). Among the Kimberley Aboriginal population, notification rates were highest in the 15–19-years age group (1816 per 100 000 population), and the notification rate ratio of Aboriginal to non-Aboriginal residents of the Kimberley region was 11.5 (141/17 153 v 12/16 851).
Among the outbreak patients, 67% (102/153) had received at least one dose of vaccine: 52% (80/153) had received two doses, and 14% (22/153) had received only one dose (Box 3). Most patients under 25 years of age (88%, 92/105) had a documented history of receiving at least one dose of vaccine, with 94% (32/34) of patients in the 15–19-years age group having this history (Box 3). Vaccine efficacy for Aboriginal people under 15 years of age, calculated by the screening method, was estimated to be 56% for full vaccination, based on an estimated 90% vaccine coverage.
Of the outbreak cases, 94% (144/153) were laboratory confirmed, and the remaining nine were diagnosed based on clinical criteria combined with an epidemiological link to a laboratory-confirmed case. Investigations performed were: serological testing, 48% of cases (73/153); polymerase chain reaction (PCR) testing of throat swabs, 93% (142/153); and both serological and PCR testing, 44% (68/153) (Box 4). Serological tests gave discordant results (IgG-positive, IgM-negative) in 68% of cases tested (50/73), but all patients who were IgM-negative underwent PCR testing, with positive results for mumps virus in 94% (47/50). Genotype J virus was identified in 20 of the cultured mumps isolates.
Mumps outbreaks have been reported recently in other highly vaccinated populations, with similar patterns of age distribution.10-14 An outbreak in the United States in 2006 (genotype G) predominantly affected 18–24-year-old students, 51% of whom had received two doses of vaccine.10 In Canada, a mumps outbreak in 2007 (genotype G) predominantly affected the 20–29-years age group (58% of cases); most patients had received at least one dose of vaccine.11 Similar outbreaks have been reported recently in Moldova,12 the Czech Republic13 and South Korea.14 An outbreak in the United Kingdom in 2005 (genotype G) affected 56 000 people, but differed in that only a minority of patients had received two vaccine doses.15 Recent outbreaks in Ireland16 and in the Netherlands (genotype D)17 also affected populations with relatively low mumps-vaccine coverage.
There are several possible causes of the limited effectiveness of the mumps vaccine in outbreak settings.18 The estimated herd-immunity threshold for mumps is 88%–92% in non-outbreak settings, and may be higher in high-risk exposure settings.19 Vaccine effectiveness is about 80% after one dose2,3,20 and about 88% after two.20,21 Even if there were 95% coverage with 95% vaccine effectiveness, population immunity would be 90%, barely reaching the level needed for herd immunity. Coverage data for the Kimberley region are not available for the 1990s, but for 2002–2008, completion of two doses of MMR vaccine by the age of 6 years varied in the order of 80%–95%.7 High vaccination coverage in the outbreak area is supported by the finding that all 73 patients who underwent serological testing were positive for mumps IgG.
It is unclear why this outbreak was confined to Aboriginal residents of the Kimberley region. The persistence of the epidemic in Broome accords with the movement of Aboriginal people from remote communities and smaller towns to this major commercial, social and medical hub of the Kimberley region. As among college students involved in outbreaks in other countries,11,14,22 the social dynamics within Aboriginal communities include overcrowded living arrangements, extensive travel between com-munities and failure to adhere to isolation requests. This may have facilitated disease transmission, including transmission from individuals with subclinical and mild vaccine-modified disease.10,23
There could be differences in the immunogenicity of the mumps vaccine in Aboriginal compared with non-Aboriginal people, because of either genetic or general health differences. Aboriginal people have significantly poorer living conditions and health indicators, which may affect their ability to mount an adequate immune response. However, there is no general indication of inability to respond to other routine childhood vaccines. Rates of most vaccine-preventable diseases targeted by the childhood immunisation program differ little between Aboriginal and non-Aboriginal children; the differences that do exist may reflect failure to vaccinate, rather than vaccine failure.24,25
Secondary vaccine failure is another possible explanation for this outbreak. The duration of post-vaccination immunity is unknown, and there are no data correlating specific antibody titre with susceptibility to mumps.26 Immunity may wane in the absence of continued natural exposure.10 In the early vaccine era, the vaccine effect was boosted by asymptomatic reinfection with circulating wild-type virus. As the amount of wild-type virus is reduced by increasing vaccination coverage, the opportunity for it to boost immunity decreases.3 In Australia, those born after 1990 experienced higher levels of vaccination coverage and less exposure to wild-type virus.4,27 Notification data indicate virtually no mumps transmission in the years preceding this outbreak.
Mumps vaccine-induced immunity may be less effective against heterologous strains, especially with waning levels of neutralising antibody.10,28,29 The current mumps vaccine strain is of genotype A lineage, while the virus identified in this outbreak was genotype J. Previous neutralisation tests have shown that vaccination with a mumps vaccine containing only one virus genotype may not protect against infection with different genotypes.29 It remains unclear whether the genotype J mumps virus responsible for this outbreak is a recent incursion to Australia. Genotype J was identified in Japan in 199430,31 and in the United Kingdom in 199732 as a sporadic virus. Prospective monitoring of the genotypes of circulating mumps virus in Australia and elsewhere will be important in investigations of both endemic and epidemic mumps.
This study has limitations. Many of the affected communities are remote. A significant number of cases might have been missed, because they were asymptomatic or mild, or because of limited access to medical care and testing. Data on disease severity were not consistently collected, and it was difficult to locate vaccination records for individuals aged over 25 years. There were also difficulties interpreting results of serological tests, as mumps IgM may be transient or undetectable in infected individuals who have received mumps vaccine.3 After the outbreak was recognised, clinicians were encouraged to take swabs for PCR testing, and ultimately a remarkable 94% of cases were laboratory confirmed.
1 Epidemic curve of notified mumps cases associated with the 2007–2008 Kimberley outbreak, by date of illness onset and place of residence
2 Age distribution of patients and age-specific notification rates for Kimberley mumps outbreak
* Aboriginal population of Kimberley region used as denominator. |
|||||||||||||||
- Revle D Bangor-Jones1
- Gary K Dowse1
- Carolien M Giele1
- Paul G van Buynder1
- Meredith M Hodge2
- Mary M Whitty3
- 1 Communicable Disease Control Directorate, WA Department of Health, Perth, WA.
- 2 PathWest Laboratory Medicine WA, Perth, WA.
- 3 Kimberley Public Health Unit, Broome, WA.
We thank Dr Carole Reeve (Public Health Medical Officer, Kimberley Public Health Unit, Broome), Dr Shelley Deeks (formerly, Deputy Director, Surveillance, National Centre for Immunisation Research and Surveillance), the staff of the Kimberley Population Health Unit, and laboratory staff at PathWest.
None identified.
- 1. National Health and Medical Research Council, Department of Health and Ageing. The Australian immunisation handbook. 9th ed. Canberra: Australian Government, 2008.
- 2. Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. 11th ed. Washington, DC: Public Health Foundation, 2009. http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/mumps.pdf (accessed Jul 2009, link updated Sep 2009).
- 3. Hviid A, Rubin S, Muhlemann K. Mumps. Lancet 2008; 371: 932-944.
- 4. Aratchige P, McIntyre P, Quinn H, Gilbert G. Recent increases in mumps incidence in Australia: the “forgotten” age group in the 1998 Measles Control Campaign. Med J Aust 2008; 189: 434-437. <MJA full text>
- 5. Codde DJ. Rates calculator. Version 9.3.1. Perth: Epidemiology and Geographic Information System Branch, Department of Health Western Australia, 2007.
- 6. Coleman K, Markey P, Krause V. Mumps in the NT. NT Dis Control Bull 2008; 15 (4): 11-17. http://www.health.nt.gov.au/library/scripts/objectifyMedia.aspx?file=pdf/32/88.pdf&siteID=1&str_title=Bulletin December 2008.pdf (accessed Jul 2009).
- 7. Medicare Australia. Australian Childhood Immunisation Register (ACIR) statistics. http://www.medicareaustralia.gov.au/provider/patients/acir/statistics.jsp (accessed Jul 2009).
- 8. Sheehan J. An information system for rural community services. In: Chater B, Malko K, editors. A fair go for rural health — forward together. Proceedings of the 2nd National Rural Health Conference; 1993 Feb 12–14; Armidale, NSW.
- 9. Gail M, Benichou J. Encyclopedia of epidemiologic methods. Chichester, UK: John Wiley, 2000.
- 10. Dayan G, Quinslink P, Parker A, et al. Recent resurgence of mumps in the United States. N Engl J Med 2008; 358: 1580-1589.
- 11. Public Health Agency of Canada. Information for health professionals. Mumps in Canada, 2007. http://www.phac-aspc.gc.ca/mumps-oreillons/prof-eng.php (accessed Jul 2009).
- 12. Bernard H, Schwarz N, Melnic A, et al. Mumps outbreak ongoing since October 2007 in the Republic of Moldova. Euro Surveill 2008; 13 (13). pii: 8079.
- 13. Boxall N, Kubinyiova M, Prikazsky V, et al. An increase in the number of mumps cases in the Czech Republic, 2005-2006. Euro Surveill 2008; 13 (16). pii: 18842.
- 14. Park D, Nam M, Kim J, et al. Mumps outbreak in a highly vaccinated school population: assessment of secondary vaccine failure using IgG avidity measurements. Vaccine 2007; 25: 4665-4670.
- 15. Savage E, Ramsay M, White J, et al. Mumps outbreaks across England and Wales in 2004: observational study. BMJ 2005; 330: 1119-1120.
- 16. Gee S, O’Flanagan D, Fitzgerald M, Cotter S. Mumps in Ireland, 2004–2008. Euro Surveill 2008; 13 (18). pii: 18857.
- 17. Karagiannis I, van Lier A, van Binnendijk R, et al. Mumps in a community with low vaccination coverage in the Netherlands. Euro Surveill 2008; 13 (24). pii: 18901.
- 18. Fine P, Zell E. Outbreaks in highly vaccinated populations: implications for studies of vaccine performance. Am J Epidemiol 1994; 139: 77-90.
- 19. Anderson R, May R. Vaccination and herd immunity to infectious diseases. Nature 1985; 318: 323-329.
- 20. Schaffzin J, Pollock L, Schulte C, et al. Effectiveness of previous mumps vaccination during a summer camp outbreak. Pediatrics 2007; 120: e862-e868.
- 21. Harling R, White J, Ramsay M, et al. The effectiveness of the mumps component of the MMR vaccine: a case control study. Vaccine 2005; 23: 4070-4074.
- 22. Savage E, Brown D, Ramsay M. Mumps epidemic: United Kingdom, 2004-2005. MMWR Morb Mortal Wkly Rep 2006; 55: 173-175.
- 23. Senanayake S. Mumps: a resurgent disease with protean manifestations. Med J Aust 2008; 189: 456-459. <MJA full text>
- 24. McIntyre P, Menzies R. Immunisation: reducing health inequality for Indigenous Australians [editorial]. Med J Aust 2005; 182: 207-208. <MJA full text>
- 25. Menzies R, Turnour C, Chiu C, McIntyre P. Vaccine preventable diseases and vaccination coverage in Aboriginal and Torres Strait Islander people, Australia, 2003 to 2006. Commun Dis Intell 2008; 32 Suppl: 2-67.
- 26. National Advisory Committee on Immunization. Statement on mumps vaccine. An Advisory Committee Statement. Can Commun Dis Rep 2007; 33: 1-10. http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/07pdf/acs33-08.pdf (accessed Feb 2009).
- 27. Gidding H, Burgess M, Kempe A. A short history of vaccination in Australia. Med J Aust 2001; 174: 37-40.
- 28. Rubin S, Mauldin J, Chumakov K, et al. Serological and phylogenetic evidence of monotypic immune responses to different mumps virus strains. Vaccine 2006; 24: 2662-2668.
- 29. Nöjd J, Tecle T, Samuelsson A, Orvell C. Mumps virus neutralizing antibodies do not protect against reinfection with a heterologous mumps virus genotype. Vaccine 2001; 19: 1727-1731.
- 30. Jin L, Rima B, Brown D, et al. Proposal for genetic characterisation of wild-type mumps strains: preliminary standardisation of the nomenclature. Arch Virol 2005; 150: 1903-1909.
- 31. Inou Y, Nakayama T, Yoshida N, et al. Molecular epidemiology of mumps virus in Japan and proposal of two new genotypes. J Med Virol 2004; 73: 97-104.
- 32. Jin L, Brown D, Litton P. Genetic diversity of mumps virus in oral fluid specimens: application to mumps epidemiological study. J Infect Dis 2004; 189: 1001-1008.





Abstract
Objective: To describe a prolonged outbreak of mumps in the Kimberley region of Western Australia in 2007–2008.
Design: Descriptive analysis of all mumps cases notified to the WA Notifiable Infectious Diseases Database for the period 1 July 2007 to 30 June 2008.
Main outcome measures: Notified cases of mumps by patients’ place of residence, age, Indigenous or non-Indigenous ethnicity, vaccination status and method of diagnosis.
Results: 84% (153/183) of mumps notifications in WA over the study period occurred in the Kimberley region or were directly linked to Kimberley cases. Median age of patients was 18 years (range, 2–63 years), and 54% of patients were aged less than 20 years. Almost all (92%) were Australian Aboriginal people; 67% (102/153) had received at least one dose of mumps vaccine, and 52% had received two doses. The highest notification rate (1816 cases per 100 000 population) was in the Aboriginal 15–19-years age group, and 92% of these patients had received at least one dose of mumps vaccine. Almost all outbreak cases (94%) were laboratory confirmed. Genotyping was performed on 20 mumps virus isolates: all were genotype J.
Conclusion: A prolonged outbreak of mumps occurred in a well defined, highly vaccinated, predominantly young Aboriginal population in the remote Kimberley region of WA. This outbreak raises questions about the effectiveness and scheduling of the current vaccine (which is genotype A-derived), especially for Aboriginal people. Surveillance of circulating mumps virus genotypes and neutralisation studies will help in evaluating the protection provided by the current vaccine against genotypically different strains.