Reports from the national Australian Diabetes, Obesity and Lifestyle (AusDiab) study have previously been used to highlight the alarming levels of overweight and obesity now prevalent among adult Australians.1 Overweight and obesity are increasingly common,2 and contribute significantly to multiple adverse health outcomes, including type 2 diabetes, cardiovascular disease (CVD), the metabolic syndrome, hypertension and dyslipidaemia, as well as premature mortality.3-6
The methods of the AusDiab study have been described in detail elsewhere.7,8 The AusDiab study was a nationwide, population-based, stratified cluster survey of 11 247 adults (44.9% men) aged 25 years or older conducted between May 1999 and December 2000 (the response rate was 55.3% of those completing a household interview and estimated to be 37% of the eligible population). Between June 2004 and December 2005, 6537 of the 10 788 eligible participants (60.6%) returned for a follow-up physical examination. After excluding 285 participants aged over 75 years (because of the established lack of association between obesity and many health outcomes in older people),9 42 pregnant women, and 138 participants for whom there were no data on waist circumference, 6072 participants (54.7% women) were available for analysis of incident diabetes, the metabolic syndrome and its components.10 Responders to the follow-up physical examination were more likely than non-responders to be non-smokers, tertiary-educated, married and to speak English at home.8
Waist circumference was measured twice, halfway between the lower border of the ribs and the iliac crest on a horizontal plane. If measurements varied by > 2 cm, a third was taken; the mean of the two closest measurements was calculated. A 75 g oral glucose tolerance test was conducted at baseline and follow-up surveys in all non-pregnant participants not using insulin or taking oral hypoglycaemic drugs. Biochemical parameters, height, weight and blood pressure were measured as previously described.8
Diabetes was classified according to WHO criteria,11,12 and the metabolic syndrome was defined according to the International Diabetes Federation (IDF) definition.13 Hypertension and cut-off points for elevated triglyceride levels and low levels of high-density lipoprotein (HDL) cholesterol were as described in the IDF definition of the metabolic syndrome. WHO waist circumference cut-off points representing “increased” and “substantially increased” risk of obesity-associated metabolic complications in Europids were used to represent “overweight” (men, 94 cm; women, 80 cm) and “obesity” (men, 102 cm; women, 88 cm), respectively.14 One-week recall of leisure-time physical activity was assessed with the Active Australia Survey (administered by the interviewer), which has been shown to have good test–retest reliability.15,16 Self-reported television-viewing time over the previous week, smoking status and the level of education achieved were assessed with a questionnaire administered by the interviewer.
Incident myocardial infarction was ascertained by physician adjudication of medical records according to WHO MONICA (Multinational MONItoring of trends and determinants in CArdiovascular disease) criteria for myocardial infarction,17 as previously described.18 These methods have been validated against a hospital morbidity database.18
Death was ascertained by linking the AusDiab cohort to the Australian National Death Index, as described previously.19 The accuracy of the National Death Index for ascertainment of vital status has been established.20 The follow-up period for all-cause mortality was to the date of death or 30 April 2008, whichever occurred first. All 107 of those who died within 2 years of the baseline survey were excluded. The average mortality follow-up was 95.8 months, with 316 deaths occurring during the follow-up period.
To test for linear trends in means and linear associations in proportions of baseline characteristics among normal, overweight and obese groups, one-way analysis of variance — with a linear polynomial term — and χ2 tests for linear trend, respectively, were used. Age-adjusted logistic regression was used to calculate odds ratios (ORs) for incident diabetes, elevated triglyceride levels, hypertension, the metabolic syndrome and reduced HDL cholesterol levels, comparing those classified as overweight or obese with those classified as normal at baseline, and for quintiles of waist circumference. The population attributable fraction (AFp) was calculated for men and women using the following formula:21
where p is the sex-specific proportion of obesity in the baseline AusDiab cohort.1 Risk ratios (RRs) were estimated from the calculated ORs and hazard ratios (HRs) for incident events using the method of Zhang and Yu.22 Cox proportional hazard models were used to estimate all-cause mortality HRs for quintiles of waist circumference and waist-to-hip ratio (included because it was shown in two other Australian cohorts to be more strongly associated with mortality than was waist circumference)23,24 and to estimate HRs for myocardial infarction among those classified as overweight or obese compared with those classified as normal. For mortality analyses, the lowest adjusted risk for mortality was observed in the second quintile for waist-to-hip ratio. This group was therefore chosen as the reference group, with the higher mortality risk in the first quintile most likely the result of weight-loss inducing conditions such as respiratory diseases and cancer. Proportionality of hazards was assessed with log–log plots of the relative hazards by time and Kaplan–Meier plots of the observed versus predicted survival curves, using Stata 10 (StataCorp, College Station, Tex, USA). All other analyses were conducted with SPSS 15.0 (SPSS Inc, Chicago, Ill, USA).
Baseline physiological and demographic characteristics of the cohort, stratified by baseline waist-circumference categories, are shown in Box 1. Strong linear associations (P < 0.001) were seen in both men and women between abdominal obesity and: education; physical activity; television viewing; all lipid, glucose and blood pressure parameters; type 2 diabetes; the metabolic syndrome; and history of CVD.
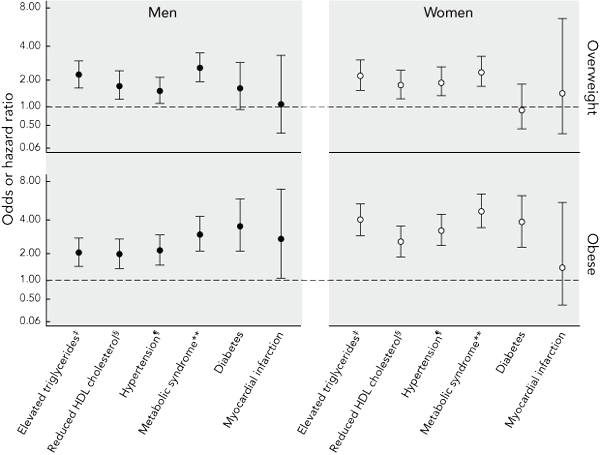
Abdominal obesity-related adjusted ORs for the development of various clinical outcomes are shown in Box 2. Among those classified as obese, compared with those with a normal waist circumference, the risk of type 2 diabetes, dyslipidaemia, hypertension and the metabolic syndrome was increased by between two and five times. The risk of myocardial infarction was similarly increased in men (HR, 2.75; 95% CI, 1.08–7.03; P = 0.035), but not women (HR, 1.43; 95% CI, 0.37–5.50; P = 0.6). Those with a waist circumference in the overweight range were at increased risk of the metabolic syndrome and its three components (hypertension, elevated triglyceride level and a low level of HDL cholesterol). Risk for type 2 diabetes in men only, and for myocardial infarction in women only, was increased in the overweight; however, this did not reach statistical significance. As a larger cohort was available for analysis of myocardial infarction than other outcomes, we repeated this analysis after excluding the 2069 participants who only participated in the phone-based follow-up of myocardial infarction (who accounted for 13 of the 45 myocardial infarction events). HRs for overweight and obesity, respectively, were 1.8 (95% CI, 0.3–10.6) and 2.1 (95% CI, 0.4–9.9) in women, and 0.7 (95% CI, 0.2–2.6) and 1.9 (95% CI, 0.7–5.1) in men (all P > 0.05).
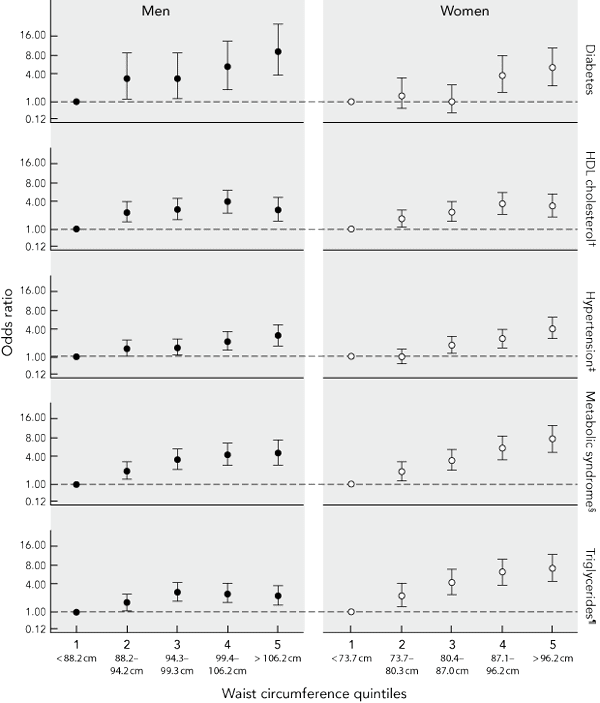
To assess risk over the continuum of waist circumference, ORs for incident type 2 diabetes, the metabolic syndrome, hypertension and dyslipidaemia were plotted against quintiles of waist circumference. Myocardial infarction was not included because of the small number of cases in each sex-specific waist circumference quintile. Increases in risk begin below the cut-off point for overweight (Box 3), with statistically significant increases in the odds of all outcomes in men, and with elevated triglyceride levels, reduced levels of HDL cholesterol and the metabolic syndrome in women being observed by the second quintile of waist circumference (73.7–80.3 cm in women; 88.2–94.2 cm in men).
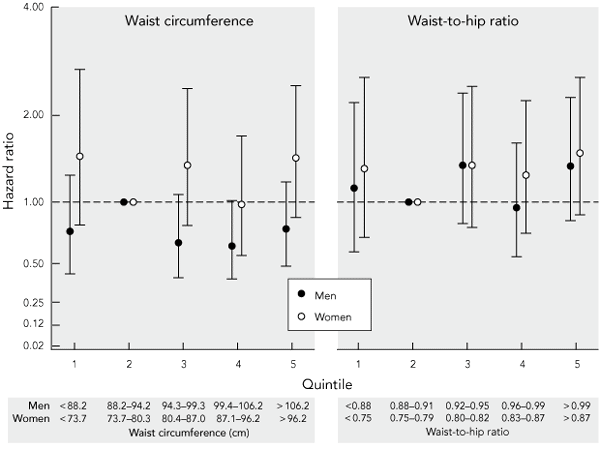
Hazard ratios for all-cause mortality over 8 years of follow-up were plotted against quintiles of waist circumference and waist-to-hip ratio, adjusted for age, history of CVD, non-skin cancer and smoking status (Box 4). Even though a weak J-shaped relationship between increasing levels of obesity and mortality was evident for waist-to-hip ratio in women, this did not reach statistical significance in any quintile. No trend of increasing risk of death with increasing obesity was evident for waist circumference in women, or for waist circumference or waist-to-hip ratio in men. Similar results were obtained after excluding smokers and those reporting a history of CVD or cancer; or when deaths in only the first year (rather than 2 years) of follow-up were excluded or when no deaths were excluded.
The obesity-related population attributable fraction was estimated for each non-fatal outcome (Box 5). This was highest for type 2 diabetes (47.4% in women, 38.0% in men); similar for elevated triglyceride levels, reduced levels of HDL cholesterol and hypertension (all over 30% in women and around 17% in men); and, for myocardial infarction, was higher in men than women (31.9% v 12.8%).
The data for the figure in Box 2, as well as the risk per unit of body mass index (BMI) or per centimetre of waist circumference, are provided in tabular form in the Appendix.
Our findings provide further evidence of the serious negative health effects that are a consequence of the high and increasing rates of overweight and obesity in Australia. Previous reports from the AusDiab study showed that in 2000, 60% of adult Australians were overweight or obese,1 with the prevalence of obesity in Australia among the highest of any developed country.25 Follow-up of the AusDiab cohort has now allowed us to report on the impact of what has been described as an obesity epidemic. The results presented here confirm that abdominal overweight and, more particularly, obesity are significant risk factors for multiple negative health outcomes, and demonstrate the serious health consequences of the obesogenic environment in which we live.
We could not fully cover the spectrum of ill health associated with obesity, as several conditions, including osteoarthritis, cancers, chronic obstructive pulmonary disease, gall bladder disease, sleep apnoea and depression, were not assessed in the AusDiab study.6,14 However, we do include four of the top five conditions for which obesity resulted in disability-adjusted life-years lost in the 1996 Australian Burden of Disease and Injury Study.26 Indeed, CVD, hypertension and type 2 diabetes were responsible for 68% of the obesity-related burden of disease. A recent report estimated the annual direct and indirect financial costs of obesity in Australia to be $3.8 billion, with over half of this borne by government and society.27
A WHO report into the consequences of obesity highlighted a lack of relative-risk data as a major evidence gap that prevents more accurate burden-of-disease estimates.14 Obesity-related risk is related to the demographic, behavioural and biomedical risk factor profile of the population, which differs between countries and over time. It is therefore difficult to extrapolate estimates from other populations to the Australian situation. The results presented here help to address this gap in the evidence, and are the first estimates of obesity-related relative risk for these conditions from a national Australian sample. Furthermore, they provide valuable evidence with which to more precisely calculate the total economic and health burden attributable to obesity, and to inform initiatives for addressing the already high levels of obesity present in Australia.
The population attributable fraction estimates presented require careful interpretation. They effectively compare the incidences of outcomes observed in the AusDiab sample with those in a hypothetical population in which obesity is totally absent. Since no intervention currently exists to eliminate obesity, they must be considered purely theoretical.28 Indeed, national obesity rates have not fallen as the result of targeted interventions in any country.29 A more detailed case study of the impact of obesity reduction interventions is obviously required. The risk estimates presented here will help to inform such endeavours in the Australian context.
Although BMI is the most frequently reported index of obesity, and a measure routinely used in WHO obesity surveillance initiatives, the recently announced Australian national obesity campaign (“Measure Up”) is based on the promotion of waist circumference measurement to identify obesity.30 Waist circumference is easily measured and has been shown to be both a better indicator of abdominal adiposity and a stronger predictor of many health outcomes than is BMI.5,14,31 Evidence-based cut-off points for waist circumference in different ethnic groups are lacking, and therefore those used in this report are appropriate only for Europid populations. For comparative purposes, analyses using BMI to categorise obesity are presented in the Appendix. We included waist-to-hip ratio in the analysis of mortality because it has been shown in two other population-based Australian cohorts to be more closely associated with mortality than was waist circumference.23,24 This trend was also present in the AusDiab cohort, even though the increased HRs did not reach statistical significance, most likely because the follow-up period was only 8 years.
It is important to interpret this report in the context of the inherent limitations of the survey. Firstly, the risks associated with lesser degrees of overweight and obesity, particularly for myocardial infarction and mortality, may not become apparent without considerably longer follow-up than the 5 years (and 8 years for mortality) used here. Other appropriately conducted and analysed studies with longer follow-up and more deaths have shown a strong and independent relationship between abdominal obesity and mortality.24,32,33 Secondly, rates of non-response to the surveys at baseline and at follow-up mean that the results are from a population-based, but not necessarily representative, sample of Australians. Finally, small numbers of Indigenous Australians in the sample mean that obesity-related risks for this population cannot be estimated.
Previous reports from the AusDiab study have shown strong associations between abdominal obesity and time spent in both physical activity and television viewing,1 and have also shown the effects of these behaviours and time spent being sedentary on markers of cardiometabolic risk.34-36 Tackling the obesity epidemic will require environmental and policy initiatives that provide realistic and achievable opportunities for Australians to be more active, to avoid too much time spent sitting and to avoid obesogenic food environments.37 A recent report from the Obesity Working Group of the national Preventative Health Taskforce has highlighted the multisectoral approach required to achieve the goal of preventing unhealthy weight gain in Australia.29
1 Cross-sectional associations of health and demographic characteristics with sex and waist circumference categories at baseline among non-pregnant adults aged ≤ 75 years who participated in both baseline (1999–2000) and follow-up (2004–2005) Australian Diabetes, Obesity and Lifestyle (AusDiab) surveys, and for whom there were waist circumference data*
2 Sex-specific adjusted* odds or hazard ratios† (and 95% confidence intervals) among Australian adults aged 25–75 years for the development of various clinical outcomes over 5 years in those classified as obese or overweight on the basis of waist circumference at baseline, compared with those with a normal waist circumference at baseline
3 Sex-specific adjusted* odds ratios and 95% confidence intervals for the development of various clinical outcomes and biomedical markers of cardiometabolic risk over 5 years by quintiles of waist circumference at baseline among Australian adults aged 25–75 years
4 Adjusted* hazard ratios and 95% confidence intervals for all-cause mortality over 8 years by quintiles of baseline waist-to-hip ratio and waist circumference in Australian adults aged 25–75 years
 | |||||||||||||||
5 Estimated fraction of incident outcomes that would not have occurred in the Australian Diabetes, Obesity and Lifestyle (AusDiab) study population if no obesity was present (population attributable fraction)
Population attributable fraction |
|||||||||||||||
HDL = high-density lipoprotein. |
|||||||||||||||
Appendix Sex-specific adjusted* odds ratios or hazard ratios† among Australian adults aged 25–75 years for the development of various clinical outcomes over 5 years in those classified as obese or overweight at baseline compared with those who at baseline had a normal waist circumference and/or body mass index (BMI), and per unit of BMI or per centimetre of waist circumference
|
NB: The Appendix is not available in the print or pdf version of this article. |
|||||||||||||||
Received 3 December 2008, accepted 1 April 2009
- Adrian J Cameron1
- David W Dunstan1
- Neville Owen2
- Paul Z Zimmet1
- Elizabeth L M Barr1
- Andrew M Tonkin3
- Dianna J Magliano1
- Shirley G Murray1
- Timothy A Welborn4
- Jonathan E Shaw1
- 1 Baker IDI Heart and Diabetes Institute, Melbourne, VIC.
- 2 School of Population Health, University of Queensland, Brisbane, QLD.
- 3 Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, VIC.
- 4 University of Western Australia, Perth, WA.
We are most grateful to the many people involved in organising and conducting the AusDiab study, and especially to the study participants for volunteering their valuable time. Adrian Cameron is supported by a postgraduate research scholarship (PP04M1794) from the National Heart Foundation of Australia. Elizabeth Barr is supported by a National Health and Medical Research Council (NHMRC) (379305)/National Heart Foundation of Australia (PP05M2346) joint postgraduate scholarship. David Dunstan is supported by Victorian Health Promotion Foundation Public Health Research Fellowships. Neville Owen is supported by a Queensland Health Core Research Infrastructure grant and by NHMRC Program Grant funding (301200). The AusDiab study was supported by a project grant from the NHMRC (233200). Collection and adjudication of non-fatal CVD outcomes was supported by a National Heart Foundation of Australia Research Grant in Aid (RES 17-01 2005). The AusDiab study, co-coordinated by the Baker IDI Heart and Diabetes Institute, gratefully acknowledges the generous support given by: Australian Government Department of Health and Ageing, Abbott Australasia, Alphapharm, AstraZeneca, Aventis Pharma, Bristol-Myers Squibb, City Health Centre Diabetes Service — Canberra, Diabetes Australia, Healthy Living NT, Eli Lilly Australia, Estate of the late Edward Wilson, GlaxoSmithKline, Jack Brockhoff Foundation, Janssen-Cilag, Kidney Health Australia, Marian & E.H. Flack Trust, Menzies Research Institute, Merck Sharp & Dohme, New South Wales Health, Northern Territory Department of Health and Families, Novartis, Novo Nordisk, Pfizer, Pratt Foundation, Queensland Health, Roche Diagnostics Australia, Royal Prince Alfred Hospital (Sydney), Sanofi-Synthelabo, South Australia Health, Tasmanian Department of Health and Human Services, Victorian Department of Human Services, and Western Australia Health.
None identified.
- 1. Cameron AJ, Welborn TA, Zimmet PZ, et al. Overweight and obesity in Australia: the 1999–2000 Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Med J Aust 2003; 178: 427-432. <MJA full text>
- 2. Australian Bureau of Statistics. National health survey: summary of results, 2004–05. Canberra: ABS, 2006. (ABS Cat. No. 4364.0.) http://www.abs.gov.au/AUSSTATS/abs@.nsf/Details Page/4364.02004-05?OpenDocument (accessed Jun 2009).
- 3. Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006; 444: 875-880.
- 4. Australian Institute of Health and Welfare, National Heart Foundation of Australia. The relationship between overweight, obesity and cardiovascular disease: a literature review prepared for the National Heart Foundation of Australia. Cardiovascular disease series no. 23. Canberra: AIHW, 2004. (AIHW Cat. No. CVD 29.) http://www.aihw.gov.au/publications/index.cfm/title/10078 (accessed Jun 2009).
- 5. Cameron AJ, Zimmet PZ. Expanding evidence for the multiple dangers of epidemic abdominal obesity. Circulation 2008; 117: 1624-1626.
- 6. Australian Institute of Health and Welfare. Chronic diseases and associated risk factors in Australia, 2006. Canberra: AIHW, 2006. (AIHW Cat. No. PHE 81.) http://www.aihw.gov.au/publications/index.cfm/title/10319 (accessed Jun 2009).
- 7. Dunstan DW, Zimmet PZ, Welborn TA, et al. The Australian Diabetes, Obesity and Lifestyle Study (AusDiab) — methods and response rates. Diabetes Res Clin Pract 2002; 57: 119-129.
- 8. Barr L, Magliano D, Zimmet P, et al. AusDiab 2005. The Australian Diabetes, Obesity and Lifestyle Study. Tracking the accelerating epidemic: its causes and outcomes. Report. Melbourne: International Diabetes Institute, 2006.
- 9. Brown CD, Higgins M, Donato KA, et al. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res 2000; 8: 605-619.
- 10. Sicree RA, Zimmet PZ, Dunstan DW, et al. Differences in height explain gender differences in the response to the oral glucose tolerance test — the AusDiab study. Diabet Med 2008; 25: 296-302.
- 11. World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO consultation. Part 1: Diagnosis and classification of diabetes mellitus. WHO/NCD/NCS/99.2. Geneva: WHO Department of Noncommunicable Disease Surveillance, 1999. http://whqlibdoc.who.int/hq/1999/WHO_NCD_NCS_99.2.pdf (accessed Jun 2009).
- 12. World Health Organization, International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Report of a WHO/IDF consultation. Geneva: WHO, 2006. http://www.who.int/diabetes/publications/Definition%20and%20diagnosis %20of%20diabetes_new.pdf (accessed Jun 2009).
- 13. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome — a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med 2006; 23: 469-480.
- 14. World Health Organization. Obesity — preventing and managing the global epidemic. Report of a WHO consultation. WHO technical report series 894. Geneva: WHO, 2000. http://whqlibdoc.who.int/trs/WHO_TRS_894.pdf (accessed Jun 2009).
- 15. Australian Institute of Health and Welfare. The Active Australia Survey. A guide and manual for implementation, analysis and reporting. Canberra: AIHW, 2003. (AIHW Cat. No. CVD 22.) http://www.aihw.gov.au/publications/cvd/aas/aas.pdf (accessed Jun 2009).
- 16. Brown WJ, Trost SG, Bauman A, et al. Test–retest reliability of four physical activity measures used in population surveys. J Sci Med Sport 2004; 7: 205-215.
- 17. World Health Organization. MONICA manual. Part IV: Event registration. Section 1: Coronary event registration data component. Geneva: WHO, 1999. http://www.ktl.fi/publications/monica/manual/part4/iv-1.htm (accessed Jun 2009).
- 18. Barr ELM, Tonkin AM, Welborn TA, et al. Validity of self-reported cardiovascular disease events in comparison to medical record adjudication and a state-wide hospital morbidity database — the AusDiab study. Intern Med J 2009; 39: 49-53.
- 19. Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 2007; 116: 151-157.
- 20. Magliano D, Liew D, Pater H, et al. Accuracy of the Australian National Death Index: comparison with adjudicated fatal outcomes among Australian participants in the Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) study. Aust N Z J Public Health 2003; 27: 649-653.
- 21. Greenland S. Applications of stratified analysis methods. In: Rothman KJ, Greenland S, editors. Modern epidemiology. Philadelphia: Lipincott-Raven, 1998.
- 22. Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998; 280: 1690-1691.
- 23. Welborn TA, Dhaliwal SS, Bennett SA. Waist–hip ratio is the dominant risk factor predicting cardiovascular death in Australia. Med J Aust 2003; 179: 580-585. <MJA full text>
- 24. Simpson JA, MacInnis RJ, Peeters A, et al. A comparison of adiposity measures as predictors of all-cause mortality: the Melbourne Collaborative Cohort Study. Obesity 2007; 15: 994-1003.
- 25. World Health Organization. The SuRF Report 2: Surveillance of chronic disease risk factors. Country-level data and comparable estimates. Geneva: WHO, 2005. https://apps.who.int/infobase/surf2/start.html (accessed Jun 2009).
- 26. Mathers C, Vos T, Stevenson C. The burden of disease and injury in Australia. Canberra: AIHW, 1999. (AIHW Cat. No. PHE 17.) http://www.aihw.gov.au/publications/index.cfm/title/5180 (accessed Jun 2009).
- 27. Access Economics. The economic costs of obesity. Report by Access Economics Pty Limited to Diabetes Australia. Canberra: Access Economics, 2006. http://www.accesseconomics.com.au/publicationsreports/showreport.php?id=102 (accessed Jun 2009).
- 28. Levine B. What does the population attributable fraction mean? Prev Chronic Dis 2007; 4: A14.
- 29. Preventative Health Taskforce. Obesity in Australia: a need for urgent action. Technical Report No. 1. Canberra: Preventative Health Taskforce, 2008. http://www.preventativehealth.org.au/internet/preventativehealth/publishing.nsf/Content/tech-obesity (accessed Jun 2009).
- 30. Roxon N. Australia measures up — national obesity campaign [media release]. 17 Oct 2008. Canberra: Minister for Health and Ageing, 2008. http://www.health.gov.au/internet/ministers/publishing.nsf/Content/mr-yr08-nr-nr137.htm (accessed Jul 2009).
- 31. Dalton M, Cameron AJ, Zimmet PZ, et al. Waist circumference, waist–hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J Intern Med 2003; 254: 1-9.
- 32. Zhang C, Rexrode KM, van Dam RM, et al. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation 2008; 117: 1658-1667.
- 33. Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med 2008; 359: 2105-2120.
- 34. Healy GN, Dunstan DW, Salmon J, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care 2008; 31: 661-666.
- 35. Healy GN, Dunstan DW, Salmon J, et al. Television time and continuous metabolic risk in physically active adults. Med Sci Sports Exerc 2008; 40: 639-645.
- 36. Dunstan DW, Salmon J, Owen N, et al. Physical activity and television viewing in relation to risk of undiagnosed abnormal glucose metabolism in adults. Diabetes Care 2004; 27: 2603-2609.
- 37. Owen N, Leslie E, Salmon J, et al. Environmental determinants of physical activity and sedentary behavior. Exerc Sport Sci Rev 2000; 28: 153-158.





Abstract
Objective: To provide an estimate of the morbidity and mortality resulting from abdominal overweight and obesity in the Australian population.
Design and setting: Prospective, national, population-based study (the Australian Diabetes, Obesity and Lifestyle [AusDiab] study).
Participants: 6072 men and women aged ≥ 25 years at study entry between May 1999 and December 2000, and aged ≤ 75 years, not pregnant and for whom there were waist circumference data at the follow-up survey between June 2004 and December 2005.
Main outcome measures: Incident health outcomes (type 2 diabetes, hypertension, dyslipidaemia, the metabolic syndrome and cardiovascular diseases) at 5 years and mortality at 8 years. Comparison of outcome measures between those classified as abdominally overweight or obese and those with a normal waist circumference at baseline, and across quintiles of waist circumference, and (for mortality only) waist-to-hip ratio.
Results: Abdominal obesity was associated with odds ratios of between 2 and 5 for incident type 2 diabetes, dyslipidaemia, hypertension and the metabolic syndrome. The risk of myocardial infarction among obese participants was similarly increased in men (hazard ratio [HR], 2.75; 95% CI, 1.08–7.03), but not women (HR, 1.43; 95% CI, 0.37–5.50). Abdominal obesity-related population attributable fractions for these outcomes ranged from 13% to 47%, and were highest for type 2 diabetes. No significant associations were observed between all-cause mortality and increasing quintiles of abdominal obesity.
Conclusions: Our findings confirm that abdominal obesity confers a considerably heightened risk for type 2 diabetes, the metabolic syndrome (as well as its components) and cardiovascular disease, and they provide important information that enables a more precise estimate of the burden of disease attributable to obesity in Australia.