Chronic and end-stage kidney disease is of epidemic proportions in indigenous populations around the world.1-4 There are no published studies describing the natural history of early chronic kidney disease (CKD) risk factors in indigenous children compared with non-indigenous children.
From February 2002 to June 2004, public primary schools in urban, coastal, rural and remote areas of New South Wales where Aboriginal people are known to live were approached regarding testing. NSW has the highest Aboriginal population in Australia. To maximise power, sampling was done to obtain equal numbers of Aboriginal and non-Aboriginal children, and in similar proportions from urban, coastal, rural and remote areas. We aimed to recruit equal numbers of boys and girls, and about equal numbers of children from each 12-month age group. All primary schools in remote communities were approached, and schools in other areas were sampled if more than 20 Aboriginal children in the relevant age range attended. We attempted to enrol all Aboriginal students from participating schools, and to match them by age and sex with a random sample of non-Aboriginal students. Aboriginal status was determined using the Australian Bureau of Statistics best practice recommendations.5
Consultation with local Aboriginal medical services was undertaken and consent was sought from community leaders before commencement of the study. Approval was obtained from the ethics committees of the Children’s Hospital at Westmead, the University of Sydney, NSW area health services, and the NSW Department of Education and Training. Informed consent was obtained for each child and, in accordance with National Health and Medical Research Council (NHMRC) guidelines,6 data were collected using a standardised form and de-identified for storage and analysis before being returned to each community after the study visit. Permission to publish data was also obtained from each community.
A morning clean-catch urine specimen was collected from each child, with dipstick analysis for haematuria and albuminuria performed on-site on fresh specimens using a Bayer Clinitek 50 analyser (Bayer Healthcare, Sydney, NSW). According to Kidney Disease Outcomes Quality Initiative (KDOQI) definitions, haematuria was defined as ≥ 25 red blood cells/μL (1+), and albuminuria as an albumin–creatinine ratio ≥ 3.4 mg/mmol.7
Birthweight was provided by the child’s parent or carer by recall or from the child’s health record. Body mass index (BMI) standard deviation z scores were calculated using height and weight and an age- and sex-adjusted program.8 Blood pressure was measured on the right arm with the child sitting, using an aneroid sphygmomanometer and the largest cuff to encircle the arm and cover at least three-quarters of the length of the upper arm.9
Standardisation of urban, coastal, rural and remote locality was performed using the Accessibility/Remoteness Index of Australia, with each subject given an index score according to postcode of residence.10 To determine the level of social and economic wellbeing of areas studied, the Socio-Economic Indexes for Areas 200111 Index of Disadvantage was applied to subjects at the level of collection district of residence. This is the smallest geographic area for which the Index is available, and includes about 200 households.
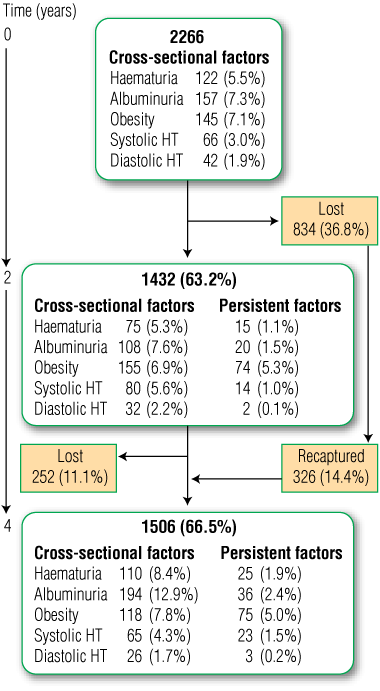
At baseline, 2266 children were enrolled from 37 primary schools in NSW (Box 1). Participation rates for both Aboriginal and non-Aboriginal children from all schools were 85%–100%. Of the 2266 children, 1248 (55.1%) were Aboriginal, 1156 (51.0%) were male, and the mean age was 8.9 years (SD, 2.0 years). Baseline characteristics have been reported in detail elsewhere.12
At 2-year follow-up, from March 2004 to December 2006, 1432 children (63.2%) were available for retesting. Of these, 773 (54.0%) were Aboriginal, 723 (50.4%) were male, and the mean age was 10.5 years (SD, 2.0 years). The 2-year follow-up results have been reported elsewhere.13 At 4-year follow-up, from February 2006 to December 2007, 1506 children (66.5%) were retested. Of these, 807 (53.6%) were Aboriginal, 768 (51.0%) were male, and the mean age was 13.3 years (SD, 3.2 years).
At final follow-up, there were proportionally more Aboriginal children in the youngest age groups and from the areas of highest isolation and disadvantage (all P < 0.001). This was also the case at baseline.12 There were more Aboriginal children with lower birthweights at final follow-up (P = 0.001).
At baseline, CKD risk factors were frequent for the group overall, ranging from 1.9% for diastolic hypertension to 7.3% for albuminuria (Box 2). There was no increased risk of baseline risk factors in Aboriginal children compared with non-Aboriginal children, except for haematuria (7.1% v 3.6%; AOR, 2.25 [95% CI, 1.37–3.69]; P = 0.001).
At 4-year follow-up, the overall prevalence of persistent risk factors was lower, ranging from 0.2% for diastolic hypertension to 5.0% for obesity (Box 2). There was no increased risk for any persistent risk factor in Aboriginal children compared with non-Aboriginal children, even after adjusting for geographic remoteness and social disadvantage.
Girls had a fourfold increased risk of persistent haematuria compared with boys (AOR, 4.31 [95% CI, 1.61–11.63]; P = 0.001) (Box 3). There was an increasing risk of persistent systolic hypertension with increasing BMI SD (trend, P < 0.001), and the highest BMI SD quartile had a 19-fold increased risk of persistent systolic hypertension compared with the lowest BMI SD quartile (AOR, 19.05 [95% CI, 2.54–43.09]; P < 0.001). There were no other predictors for persistent CKD risk factors in these children; in particular, persistent risk factors were not predicted by lower birthweight, geographic remoteness or social disadvantage.
Our cross-sectional survey of this cohort showed that baseline CKD risk factors were frequent in both Aboriginal and non-Aboriginal primary school-aged children, and that, at a single test, Aboriginal children had twice the risk of haematuria as non-Aboriginal children. Our follow-up results suggest that more than 70% of baseline urinary and blood pressure abnormalities in Aboriginal and non-Aboriginal children are transient. Semiquantitative single estimations of urinary blood and protein in children vary according to posture, illness, exercise and time of day.14 A higher rate of transient haematuria may reflect the higher incidence of transient disease seen in Indigenous children, such as post-infectious glomerulonephritis.15
Obesity was the only frequent persistent CKD risk factor in these children, although no more so in Aboriginal than non-Aboriginal children. The prevalence of persistent obesity found in our study (5%) is similar to the national rate of obesity in Australian primary school-aged children (6%).16 Increasing BMI and persistent obesity were significantly associated with persistent systolic and diastolic hypertension. These children with clustering of CKD risk factors are also particularly at risk of early-onset diabetes and cardiovascular disease.17,18
2 Prevalence of chronic kidney disease risk factors at baseline and persistent risk factors at 4-year follow-up in Aboriginal and non-Aboriginal children
Received 5 June 2008, accepted 7 October 2008
- Leigh Haysom1,2
- Rita Williams1
- Elisabeth M Hodson1,2
- Pamela A Lopez-Vargas1
- Leslie P Roy1,3
- David M Lyle3
- Jonathan C Craig1,2
- 1 Centre for Kidney Research, The Children’s Hospital at Westmead, Sydney, NSW.
- 2 School of Public Health, University of Sydney, Sydney, NSW.
- 3 University of Sydney, Sydney, NSW.
John Knight conceived and drafted the original study design. We thank the Aboriginal communities, Aboriginal medical services, schools, Aboriginal education assistants, Aboriginal area health workers, families, and the Children’s Hospital nursing staff who participated in this study; Bayer for the loan of the Clinitek 50 urinalysis machine; and the Far West Population Health Division and Maari Ma Health Aboriginal Corporation for their assistance in visiting remote communities. We would like to acknowledge the financial support provided by the NHMRC Centre for Clinical Research Excellence in Renal Medicine, the Financial Markets Foundation for Children, and the NHMRC for project grant funding and Leigh Haysom’s Training Scholarship in Indigenous Health Research. Funding bodies had no role in the study design, data collection, analysis or interpretation, or writing of this report.
None identified.
- 1. Hochman ME, Watt JP, Reid R, O’Brien KL. The prevalence and incidence of end-stage renal disease in Native American adults on the Navajo reservation. Kidney Int 2007; 71: 931-937.
- 2. Young TK, Kaufert JM, McKenzie JK, et al. Excessive burden of end-state renal disease among Canadian Indians: a national survey. Am J Public Health 1989; 79: 756-758.
- 3. Cass A, Cunningham J, Wang Z, Hoy W. Regional variation in the incidence of end-stage renal disease in Indigenous Australians. Med J Aust 2001; 175: 24-27. <MJA full text>
- 4. McDonald SP, Russ GR. Current incidence, treatment patterns and outcome of end-stage renal disease among indigenous groups in Australia and New Zealand. Nephrology (Carlton) 2003; 8: 42-48.
- 5. Australian Bureau of Statistics: 2001 Census of Population and Housing – 2001 Census Household Form. Canberra: ABS, 2001.
- 6. National Health and Medical Research Council. Values and ethics: guidelines for ethical conduct in Aboriginal and Torres Strait Islander health research. Canberra: Commonwealth of Australia, 2003. http://www.nhmrc.gov.au/PUBLICATIONS/synopses/e52syn.htm (accessed Jan 2009).
- 7. National Kidney Foundation Kidney Disease Outcomes Quality Initiative. KDOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. http://www.kidney.org/professionals/kdoqi/guidelines_ckd/toc.htm (accessed Jan 2009).
- 8. Greenacre P. Height and weight standard deviation score calculator. Sydney: Children’s Hospital at Westmead, 1997.
- 9. Arafat M, Mattoo TK. Measurement of blood pressure in children: recommendations and perceptions on cuff selection. Pediatrics 1999; 104: e30.
- 10. Australian Bureau of Statistics. Australian Indigenous geographical classification maps and census profiles, 2001. Canberra: ABS, 2002. (ABS Cat. No. 4706.0.30.001.)
- 11. Australian Bureau of Statistics: Socio-Economic Indexes for Areas (SEIFA). Canberra: ABS, 2003.
- 12. Haysom L, Williams R, Hodson E, et al. Early chronic kidney disease in Aboriginal and non-Aboriginal children: remoteness, socioeconomic disadvantage or race? Kidney Int 2007; 71: 787-794.
- 13. Haysom L, Williams R, Hodson E, et al. Risk of CKD in Australian indigenous and nonindigenous children: a population-based cohort study. Am J Kidney Dis 2009; 53: 229-237.
- 14. Yoshimoto M, Tsukahara H, Saito M, et al. Evaluation of variability of proteinuria indices. Pediatr Nephrol 1990; 4: 136-139.
- 15. Van Buynder PG, Gaggin JA, Martin D, et al. Streptococcal infection and renal disease markers in Australian Aboriginal children. Med J Aust 1992; 156: 537-540.
- 16. Booth ML, Wake M, Armstrong T, et al. The epidemiology of overweight and obesity among Australian children and adolescents, 1995–97. Aust N Z J Public Health 2001; 25: 162-169.
- 17. Lobstein T, Baur L, Uauy R; IASO International Obesity TaskForce. Obesity in children and young people: a crisis in public health. Obes Rev 2004; 5 Suppl 1: S4-S104.
- 18. National Institutes of Health. Clinical guidelines on the identification, evaluation and treatment of overweight and obesity in adults — the evidence report. Obes Res 1998; 6 Suppl 2: 51S-209S.





Abstract
Objective: To describe the natural history and risk of early chronic kidney disease (CKD) in Indigenous Australian populations.
Design, setting and participants: A prospective cohort of 2266 Aboriginal and non-Aboriginal children enrolled from primary schools throughout New South Wales from February 2002 to June 2004 and followed for 4 years.
Main outcome measures: Urinalysis, height, weight, blood pressure, birthweight and sociodemographic status at baseline and 2- and 4-year follow-up; CKD risk factors: haematuria, albuminuria, obesity, and systolic and diastolic hypertension.
Results: 2266 children (55% Aboriginal; 51% male; mean age, 8.9 years [SD, 2.0 years]) were enrolled at baseline. 1432 children (63%) were retested at 2-year follow-up, and 1506 children (67%) at 4-year follow-up. Prevalence of baseline CKD risk factors was frequent (2%–7%), but most abnormalities were transient. Besides persistent obesity (5.0%), persistence of CKD risk factors at final follow-up was low: haematuria (1.9%), albuminuria (2.4%), systolic hypertension (1.5%) and diastolic hypertension (0.2%). There was no difference in prevalence of persistent CKD risk factors between Aboriginal and non-Aboriginal children.
Conclusions: Over 4 years of follow-up, Indigenous Australian children had no increased risk for early evidence of CKD. More than 70% of baseline risk factors were transient, and persistent risk factors were uncommon. Our findings suggest the increased risk for end-stage kidney disease seen in Indigenous adults is not yet manifest in these schoolchildren, and may be potentially preventable.