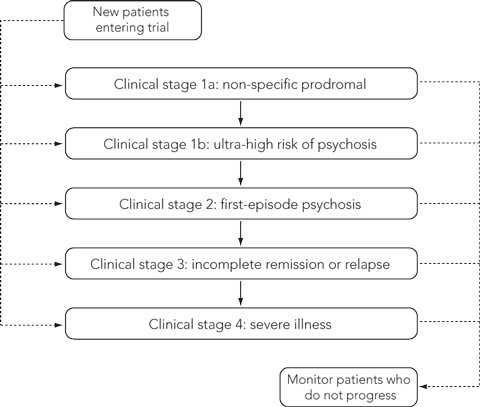
The early phases of schizophrenia and other psychotic disorders usually manifest as sustained and prolonged periods of emotional and behavioural changes. Symptoms may be diffuse, mixed, and subthreshold in pattern and severity. Recent research has shown that the prodrome of schizophrenia is indistinguishable from that of major depression.1 We contend that a kind of pluripotential prodromal stage exists. From this non-specific prodrome, there are essentially two ways a disorder can emerge. Firstly, an existing emotional or behavioural state can intensify in severity or persistence, causing distress or disturbance in the person’s life. Secondly, new subjective experiences can emerge, intensifying with distressing and disruptive effects.
We have incorporated such thinking into a clinical staging model of mental disorders.2 Within this model, the initial pluripotential prodromal stage can result in a range of outcomes, including spontaneous remission, progression to a non-psychotic disorder or development of subthreshold psychotic symptoms — the ultra-high risk (UHR) state.
In the case of psychotic disorders, there is a gradual or sudden emergence of hallucinations or delusions, which may initially appear in subthreshold or fluctuating forms. A series of studies has identified a set of clinical features that confer a UHR of proceeding to a first psychotic episode.3 First-episode psychosis is said to have developed once psychotic experiences become persistent and are at full threshold level,4 marking a point at which antipsychotic medications are indicated.3 Hence, first-episode psychosis is a practical and useful diagnostic marker, and the concept of early or first-episode psychosis has proved popular as a structural basis for service reform and investment worldwide.5
To progress this more flexible model of clinical research and related health care, much better access to health care is required for young people with emerging mental health problems. Fortunately, a change has occurred that will allow a new level of mental health clinical research in Australia. With the establishment of 30 new youth mental health service centres across Australia, headspace (http://www.headspace.org.au), the federal government’s recent major funding initiative in youth mental health, has created a unique opportunity for longitudinal cohort studies and multicentre clinical trials focused on the earliest stages of emerging mental disorders. Although headspace is in its early stages of development, several thousand young people have already gained access to multidisciplinary care, many in the subset of services led by, or with close links to, clinical research centres. We expect this will allow the progressive creation of a firm evidence base for earlier diagnosis and treatment, and lead to a better understanding of a range of mental and substance-use disorders. Given the comorbidity and dimensionality that characterise the early stages of mental disorders in young people, a “wide-angle lens” is essential to allow a focus on those who are at greatest risk of persistent and disabling forms of mental disorder.
We propose a series of larger-scale cohort studies and multicentre effectiveness trials in young people with emerging mental and substance-use disorders (Box). Early interventions need to be simpler, safer and cheaper. In fact, with disorders of slow and diverse onset, there may be an optimal time to intervene — too early may be inefficient and trivialise the concept of what a disorder is, but delay may mean the disorder becomes refractory to intervention, either for biological or psychosocial reasons. Others have proposed that cost-effectiveness is superior to current severity or disability as a triage criterion.6,7 The notion of an optimal point for intervention represents a testable hypothesis that may vary across types of disorder.
Mounting large-scale multicentre studies with a sufficient overall sample size to rapidly test new therapies will require the use of practical trials that focus on effectiveness in real-world settings, rather than narrower efficacy trials.8 From a theoretical perspective, there is a clear need to increase statistical power to conduct valid preventive and early intervention trials. We can achieve this by setting the main research boundary at the level of indicated prevention, which in turn can be accomplished by focusing on groups with multiple risk factors and early clinical changes; defining outcomes broadly and using briefer and more robust measures, with multiple exit syndromes and/or poor functioning as the key outcome to be prevented; and progressively strengthening the effectiveness of specific interventions and programs.
Recently, we have seen a shift in views of neurodevelopment, neurodegeneration and adult brain functioning, with the revelation that the central nervous system can generate and remove progenitor, glial and neuronal cells across the lifespan, not only early in life as was previously thought.9 Internal neurotrophins (eg, glutamate, dopamine, brain-derived neurotrophic factor, cytokines) and external factors (hypoxia, illicit drugs) influence whether the progenitor cells stop dividing, further differentiate, or die.9,10 Regulation of these cellular survival processes is pivotal for connectivity, synapse formation, axon migration and pruning, and can be summarised under the term synaptic plasticity.
Synaptic plasticity is influenced by the neurodevelopmental stage of the brain, and supporting brain development and protecting this plasticity provides new therapeutic options for neuroprotection that may be relevant to the changes we have identified at various stages of mental disorder, particularly during the transition to fully fledged, sustained illness. Essential fatty acids (EFAs), mood stabilisers (particularly lithium) and some second-generation antipsychotics promote neurogenesis, reduce neuroprotective proteins and protect cells from excitotoxic death. Thus, we can examine these and other potential neuroprotective agents that are relevant to preventing onset, retarding progression or improving outcome (including neuropsychological function) for patients in the early illness stages. Among the key neuroprotective mechanisms that can be influenced in treatment studies are the neurotrophic processes generally (which may be dysregulated in mental disorders),9,10 cell death, hypothalamic–pituitary–adrenal (HPA) axis function,11,12 and the control of oxidative stress.13
EFAs, such as eicosapentaenoic acid and docosahexaenoic acid, may have an adjunctive therapeutic effect in first-episode psychosis14 and a primary therapeutic effect in prepsychotic or UHR states of psychosis.15 Imaging studies suggest these benefits of EFAs may be mediated by reducing oxidative stress through an effect on glutathione metabolism.16 Because EFAs have wider efficacy in a range of syndromes17,18 and are safe and well tolerated, they are excellent candidates for trials in earlier stages of mental disorders.
Lithium is a cornerstone in the treatment of mood disorders, and it has now become clear that it has potent neuroprotective properties.19,20 Lithium treatment is associated with short-term increases in grey matter,21 and has been shown to be dramatically effective in the treatment of degenerative neurological diseases, notably amyotrophic lateral sclerosis.22 We studied use of lithium in young people at UHR of psychosis and found it to be associated with symptomatic improvement.23 We also found that lithium was associated with positive central nervous system changes, as evidenced by significant reduction in T2 relaxometry and a trend for improvements in N-acetylaspartate–creatine ratios.23 Hence, lithium could be the focus of clinical trials, particularly for use in the UHR state and subsequently in early treatment resistance.
We conducted a series of studies examining potential dysregulation of the HPA axis in response to stress and trauma in early psychosis.24-27 We found evidence of relationships between HPA dysregulation and both clinical and neurobiological variables including changes in the volume of key brain structures, notably the pituitary and the hippocampus.24 Possible interventions to be tested in clinical trials in this area include tianeptine (a novel antidepressant that affects the HPA axis),28 glucocorticoid antagonists, and psychosocial interventions to reduce and moderate the effects of stress and trauma.
Recent studies have produced evidence that N-acetylcysteine, a neuroprotective treatment believed to act via the glutathione pathway and the reduction of oxidative stress, may be effective as an adjunctive therapy in established schizophrenia and bipolar disorder.29,30 Thus, there are logical grounds for moving it forward in terms of stage of illness to earlier stages of psychotic illness.
- Patrick D McGorry1,2,3
- Alison R Yung2
- Christos Pantelis1
- Ian B Hickie4
- 1 University of Melbourne, Melbourne, VIC.
- 2 ORYGEN Youth Health, Melbourne, VIC.
- 3 ORYGEN Research Centre, University of Melbourne, Melbourne, VIC.
- 4 Brain and Mind Research Institute, University of Sydney, Sydney, NSW.
None identified.
- 1. Häfner H, Maurer K, Trendler G, et al. Schizophrenia and depression: challenging the paradigm of two separate diseases — a controlled study of schizophrenia, depression and healthy controls. Schizophr Res 2005; 77: 11-24.
- 2. McGorry PD, Purcell R, Hickie IB, et al. Clinical staging: a heuristic model for psychiatry and youth mental health. Med J Aust 2007; 187 (7 Suppl): S40-S42. <MJA full text>
- 3. Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res 2004; 67: 131-142.
- 4. Yung AR, McGorry PD, McFarlane CA, et al. Monitoring and care of young people at incipient risk of psychosis. Schizophr Bull 1996; 22: 283-303.
- 5. Edwards J, McGorry PD. Implementing early intervention in psychosis. A guide to establishing early psychosis services. London: Martin Dunitz, 2002.
- 6. Andrews G, Sanderson K, Corry J, et al. Cost-effectiveness of current and optimal treatment for schizophrenia. Br J Psychiatry 2003; 183: 427-435.
- 7. Wang PS, Simon G, Kessler RC. The economic burden of depression and the cost-effectiveness of treatment. Int J Methods Psychiatr Res 2003; 12: 22-33.
- 8. Insel TR. Beyond efficacy: the STAR*D trial. Am J Psychiatry 2006; 163: 5-7.
- 9. Berger GE, Wood S, McGorry PD. Incipient neurovulnerability and neuroprotection in early psychosis. Psychopharmacol Bull 2003; 37: 79-101.
- 10. Yao JK, Reddy RD. Membrane pathology in schizophrenia: implication for arachidonic acid signaling. ScientificWorldJournal 2002; 2: 1922-1936.
- 11. Phillips LJ, McGorry PD, Garner B, et al. Stress, the hippocampus and the hypothalamic–pituitary–adrenal axis: implications for the development of psychotic disorders. Aust N Z J Psychiatry 2006; 40: 725-741.
- 12. Corcoran C, Walker E, Huot R, et al. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull 2003; 29: 671-692.
- 13. Ng F, Berk M, Dean O, Bush A. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 2008; 11: 851-876.
- 14. Berger GE, Proffitt TM, McConchie M, et al. Ethyl-eicosapentaenoic acid in first-episode psychosis: a randomized, placebo-controlled trial. J Clin Psychiatry 2007; 68: 1867-1875.
- 15. Amminger GP, Schäfer MR, Papageorgiou K, et al. Relationship between reduced erythrocyte membrane fatty acids and transition to psychosis in ultra high risk individuals [abstract]. Schizophr Res 2008; 98 Suppl 1: 108.
- 16. Berger GE, Wood SJ, Pantelis C, et al. Implications of lipid biology for the pathogenesis of schizophrenia. Aust N Z J Psychiatry 2002; 36: 355-366.
- 17. Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis 2007; 6: 21.
- 18. Young G, Conquer J. Omega-3 fatty acids and neuropsychiatric disorders. Reprod Nutr Dev 2005; 45: 1-28.
- 19. Bachmann RF, Schloesser RJ, Gould TD, Manji HK. Mood stabilizers target cellular plasticity and resilience cascades: implications for the development of novel therapeutics. Mol Neurobiol 2005; 32: 173-202.
- 20. Jope RS. Anti-bipolar therapy: mechanism of action of lithium. Mol Psychiatry 1999; 4: 117-128.
- 21. Moore GJ, Bebchuk JM, Wilds IB, et al. Lithium-induced increase in human brain grey matter. Lancet 2000; 356: 1241-1242.
- 22. Fornai F, Longone P, Cafaro L, et al. Lithium delays progression of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 2008; 105: 2052-2057.
- 23. Berger G, Dell’Olio M, Amminger P, et al. Neuroprotection in emerging psychotic disorders. Early Interv Psychiatry 2007; 1: 114-127.
- 24. Velakoulis D, Wood SJ, Wong MTH, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry 2006; 63: 139-149.
- 25. Phillips LJ, McGorry PD, Garner B, et al. Stress, the hippocampus and the hypothalamic–pituitary–adrenal axis: implications for the development of psychotic disorders. Aust N Z J Psychiatry 2006; 40: 725-741.
- 26. Thompson KN, Phillips LJ, Komesaroff P, et al. Stress and HPA-axis functioning in young people at ultra high risk for psychosis. J Psychiatr Res 2007; 41: 561-569.
- 27. Thompson KN, Berger G, Phillips LJ, et al. HPA axis functioning associated with transition to psychosis: combined DEX/CRH test. J Psychiatr Res 2007; 41: 446-450.
- 28. Dupin N, Mailliet F, Rocher C, et al. Common efficacy of psychotropic drugs in restoring stress-induced impairment of prefrontal plasticity. Neurotox Res 2006; 10: 193-198.
- 29. Berk M, Copolov D, Dean O, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia — a double-blind, randomized, placebo-controlled trial. Biol Psychiatry 2008; 64: 361-368.
- 30. Berk M, Copolov DL, Dean O, et al. N-acetyl cysteine for depressive symptoms in bipolar disorder — a double-blind randomized placebo-controlled trial. Biol Psychiatry 2008; 64: 468-475.





Abstract
A fundamental shift in the design of clinical trials for psychotic disorders is desirable and feasible. Priority should be placed on evaluation of the efficacy of interventions targeting different phases of illness.
A range of traditional therapeutic approaches needs to be augmented by an increased emphasis on the potential benefits of informational, e-health, behavioural and neuroprotective strategies.
A new national clinical trials platform, based on headspace, the National Youth Mental Health Foundation, is outlined. It provides the opportunity for conducting large multisite clinical trials in young people with emerging major mental disorders.