Multimodal programs to change hand hygiene (HH) culture have achieved significant sustained improvements in HH compliance by health care workers and reductions in rates of infection with methicillin-resistant Staphylococcus aureus (MRSA) and other nosocomial pathogens in individual institutions in Australia and elsewhere.1-5 Although the World Health Organization and other bodies have advocated large-scale roll-outs of such programs, there are currently no data to support the efficacy of such system-wide initiatives or to describe an optimal approach.6,7 In fact, some researchers have expressed doubts about whether such programs can be effectively introduced across a range of institutions or as a statewide policy initiative, owing to their perceived dependence on enthusiastic individual champions and the complexity of developing a generic culture-change template that is suitable for multiple disparate institutions.8
After the success of a recent single-site HH culture-change program (HHCCP),1 we assessed the efficacy of a similar, but more focused, centrally coordinated 2-year pilot program in six Victorian health care institutions, and then of a 1-year program in all Victorian public hospitals (“statewide roll-out”).
Unlike previous Australian studies,1 our project focused primarily on the health care culture of compliance with appropriate HH, especially the increased use of alcohol-based hand-rub solutions (ABHRSs). After an extensive literature review, the project staff agreed that all sites should use an ABHRS product that contained at least 70% alcohol (ethanol or isopropanol), 0.5% chlorhexidine and skin emollient. Individual sites were free to choose the specific product and supplier. In addition, sites were encouraged to implement the use of alcohol-impregnated wipes for cleaning hospital equipment that was routinely shared between patients.
A generic HH culture-change training program was developed, including slide presentations, lectures, practical workshops and a training DVD explaining the standardised HH compliance tool.9 All medical and nursing leaders attended a 2-day workshop aimed at standardising and validating their accurate implementation of the tool.9 The coordination centre developed a generic guide for the stepwise introduction of the HHCCP to participating sites, which were encouraged to modify it to suit site-specific requirements and conditions.
Details of the generic HHCCP, the training manual and promotional materials, the HH credentialling package, HHCCP policy and standards and recent media releases are available from the VQC HHCCP website at http://www.health.vic.gov.au/qualitycouncil/activities/handhyg.
1. HH compliance: This was measured in pilot wards by standardised assessors at baseline (before intervention) and then every 4–6 months during the 24-month project, as described previously.1,9
2. Rates of MRSA infection: This was defined in a similar way to the previous study:1
MRSA bacteraemia: The number of patients with bacteraemia per 100 patient discharges (PD) per month in the entire institution was recorded. (“Discharges” include hospital discharges and deaths, and are referred to as “separations” in some states and territories.) A patient episode of bacteraemia was defined as a positive blood culture for MRSA, but only the first isolate for any individual patient was counted, unless at least 14 days had passed without a positive blood culture, after which any additional episode was recorded.1
MRSA isolates: The number of non-blood-culture MRSA isolates identified from clinical (non-screening) specimens per 100 PD per month in the entire institution was recorded, as described previously.1 Screening for MRSA colonisation was not a component of the HHCCP.
3. ABHRS supply data: This was defined, as previously, in terms of the number of litres of ABHRS ordered for each pilot ward per 1000 bed-days per month.1
Data collection fulfilled the National Health and Medical Research Council criteria for quality assurance in health care,10 and the HHCCP was approved as a quality care initiative at each site. Statistical analyses were undertaken as previously described.1
Seventy-seven eligible Victorian public hospitals were strongly encouraged to participate in the new program, apart from those that had participated in the pilot program or similar HHCCPs1 (Austin Health, the Royal Women’s Hospital and the Royal Children’s Hospital in Melbourne). Participation requirements were identical to those for the pilot program. The HHCCP was rolled out in two stages (Stage 1: March 2006 to April 2007; Stage 2: July 2006 to June 2007), each involving at least two rural health regions and a number of urban hospitals.
All sites used the generic HHCCP developed in the pilot program. Similarly, all sites were encouraged to use a similar ABHRS, as before, but this was not mandated and sites could choose alcohol-only products. All clinical leaders and relevant infection control staff attended a 2-day workshop, as in the pilot study. One-day workshops were also held in each of the five rural health regions, focusing on the education and roll-out initiatives to be used and on how to use the HH compliance tool. Outcome measures were identical to those used previously1,9 and in the pilot program.
Statistical analyses were undertaken as previously described.1 On the basis of our methodology and previous experience,1 we did not expect statistically significant changes in MRSA infection rates to be identified until about 30 months after the start of each program.
Overall, HH compliance increased significantly during the study period, from a mean of 21% (95% CI, 20%–22%) at baseline to 47% (95% CI, 46%–48%; range, 31%–75%) at the final assessment (P < 0.001) (Box 1). After an initial increase in HH compliance, three sites (A, B and D) showed some transient declines in compliance rates. In each case, these were related to changes in project officers. Sites with unchanged project officers experienced the most robust and sustained improvement in HH compliance. The type of ABHRS used did not influence the rates of HH compliance achieved or their sustainability.
ABHRS supply increased from a mean of 5.3 L/1000 bed-days during the 24-month pre-intervention period to a mean of 27.6 L/1000 bed-days in the final 6 months of the project, but correlated poorly with HH compliance rates, owing to marked variations in supply ordering patterns and stockpiling (Box 2).
The number of patients with MRSA bacteraemia fell significantly from a mean baseline rate of 0.05/100 PD per month (range at individual sites, 0.00–0.13) 24 months before the intervention to 0.02/100 PD per month in the last 3 months of the intervention period (P = 0.035 for trend) (Box 3). This represents a total of 65 (95% CI, 5–126) fewer patients developing MRSA bacteraemia in the six participating hospitals during the 24-month intervention period than would have been expected before the intervention.
Similarly, the total number of clinical MRSA isolates fell significantly from a mean baseline rate of 1.39/100 PD per month (range, 0.16–2.39) 24 months before the intervention to 0.73/100 PD per month 24 months after the start of the intervention (P = 0.003 for trend) (Box 4). This represents a total of 716 (95% CI, 269–1162) fewer clinical MRSA isolates identified in the six participating hospitals during the 24-month intervention period than would have been expected before the intervention. These reductions reached statistical significance 23 months after the start of the HHCCP (ie, 1 month before project completion).
All but two of the 77 eligible Victorian hospitals agreed to participate in the statewide roll-out — about half in each Stage (Box 5). Overall, the project involved sites with a total capacity of about 6154 beds.
Most sites selected Avagard or DeBug, with only a few choosing an alcohol-only ABHRS.
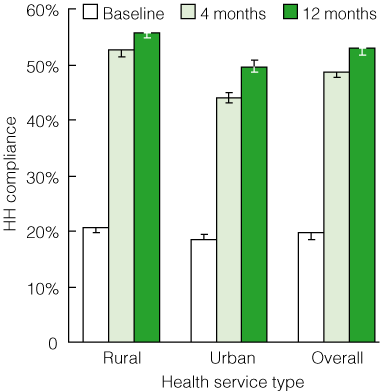
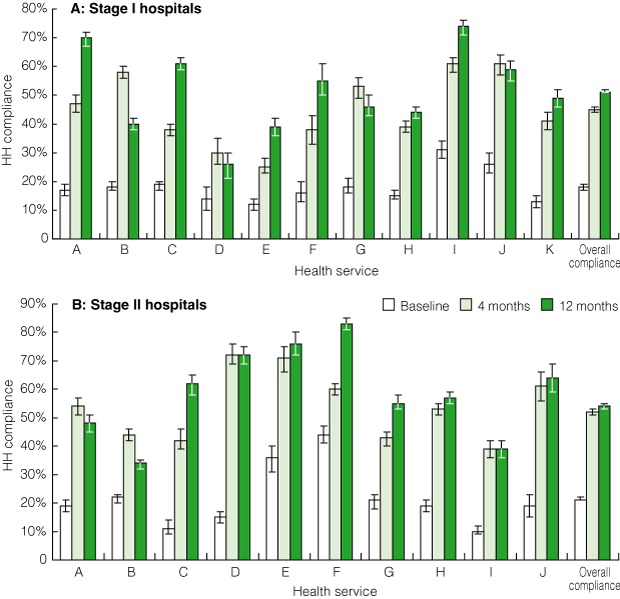
Overall, HH compliance increased significantly during the study period, from a mean rate of 20% (95% CI, 19%–20%; range, 10%–44%) at baseline to 49% (95% CI, 48%–49%; range, 25%–72%) after 4 months, to 53% (95% CI, 52%–53%; range, 26%–83%) after 11–12 months (P < 0.001 for each comparison) (Box 6). Results for Stage I and Stage II hospitals were similar (Box 7). Large rural sites demonstrated the most dramatic results: five health services achieved HH compliance rates of > 70%, with one of these achieving 83% compliance.
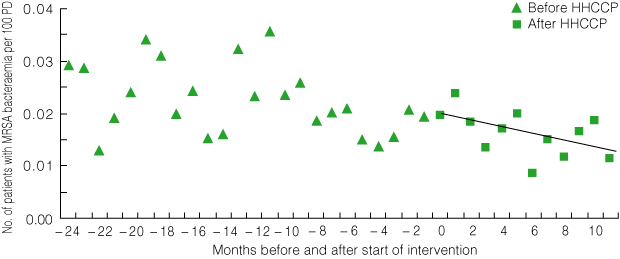
Overall, the number of patients with MRSA bacteraemia fell from a mean baseline rate of 0.03/100 PD per month to 0.01/100 PD per month 12 months after the start of the intervention (P = 0.09 for trend) (Box 8).
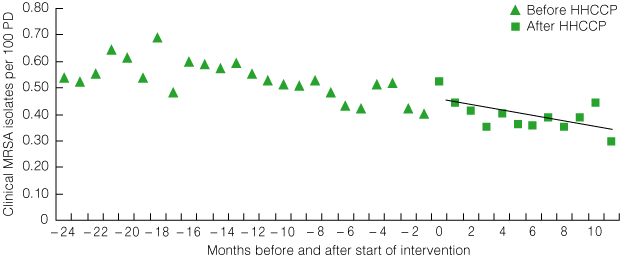
The total number of clinical MRSA isolates per month fell significantly from a mean baseline rate of 0.54 per 100 PD to 0.30 per 100 PD 12 months after the start of the intervention (P = 0.043 for trend) (Box 9). Notably, the rate of clinical MRSA isolates was declining significantly statewide before the HHCCP (P = 0.0003 for trend), and this decline continued with introduction of the HHCCP.
This is the largest multisite HHCCP both in Australia and worldwide to describe the implementation and efficacy of a generic program with hard endpoints such as HH compliance and rates of MRSA infection. As in the previous single-site study,1 we found that the pilot program resulted in significant sustained increases in overall HH compliance and significant reductions in both the number of patients with MRSA bacteraemia and the number of clinical MRSA isolates identified, standardised for hospital activity (ie, per 100 PD per month). These results are particularly notable, as the HHCCP was conducted in only two or three wards, yet significant reductions in rates of MRSA disease occurred throughout each institution. We were encouraged that these reductions in MRSA disease reached significance 23 months after the start of the HHCCP, as we had expected from our previous study1 that such improvements would not be evident until about 30 months after the start.
We believe our data demonstrate that generic multisite HHCCPs, including statewide initiatives, can be highly effective if they are carefully planned and implemented, and that previously expressed scepticism is unfounded.8 Nevertheless, the fact that some sites demonstrated a temporary reduction in their rates of HH compliance (albeit not to baseline levels) when there were delays in replacing nurse project officers highlights the fragility of such early initiatives and the difficulty in totally embedding HH culture change in organisations in short time periods. As in other culture-change initiatives, such as the wearing of seatbelts in vehicles or drink-driving campaigns, ongoing education, constant message re-enforcement and data feedback are necessary to bring about sustained change.5,6,8
Unlike the previous single-site study, we did not screen for nasal or cutaneous MRSA colonisation, as it had previously proved extremely costly, did not generally affect the management of patients (unless they were about to undergo complex surgery) and did not correlate with demonstrated reductions in MRSA infections.1 We believe our findings justify this approach, as we were able to focus the majority of funding on the intervention and collect outcome data solely on endpoints that had clear practical relevance. One exception was the recording of ABHRS supply data, which we had initially believed might be a simple surrogate for HH compliance. In fact, supply data correlated poorly with HH compliance in both the pilot program and the statewide roll-out, owing to irregular ordering patterns and stockpiling. Our experience suggests that it is unlikely to be a useful indicator. This is notable, given that some European countries plan to use rates of ABHRS usage at public hospitals as a quality and funding performance measure (Didier Pittet, Director, WHO Global Safety Challenge, Geneva, personal communication).
Our study has some limitations. Firstly, only sites that were clearly interested in participating in the HHCCP were enrolled in the pilot program, and the hospital administrations at each site gave unqualified commitment — a crucial feature of successful HHCCPs.1,2,6 Thus, we cannot be certain that similar results would be achieved in institutions in which the commitment was less enthusiastic, although the results of the statewide roll-out are encouraging. Secondly, the financial model used in our project provided clearly identified salary and incidental funds so that participating institutions could be certain about which costs were covered and which required additional institutional support. Whether other, less transparent funding models would be equally effective is uncertain. Thirdly, all but one of our pilot sites were relatively large (> 250 beds), and all had established infection control departments with full-time staff. Thus, the efficacy of HHCCPs in smaller institutions without such infection control support remains less certain — although, once again, the success of the statewide roll-out is encouraging. Fourthly, although HH compliance auditing was required only on pilot wards in both the pilot program and the statewide roll-out, it is likely that the initiatives undertaken in these areas had a beneficial effect in other wards. Finally, we cannot be certain that the improvements we observed in the statewide roll-out are related entirely to our HHCCP. For instance, on 6 September 2006 (3–6 months after the start of the statewide roll-out), the Victorian minister for health announced that all visitors to hospitals would be encouraged to use ABHRS on entry and departure. This announcement appeared to increase the public profile of the HHCCP and may have influenced the behaviour of some health care workers and visitors.
As in previous studies,1,2 our results suggest that an active HHCCP, in combination with widespread availability of ABHRS in clinical areas and targeted education of health care workers, can produce dramatic and sustained improvement in HH compliance and statistically significant reductions in MRSA disease after only 1–2 years. Our data suggest that such HHCCPs represent the single most effective means of reducing the burden of MRSA disease in Australian hospitals. Consideration of a national roll-out of such HHCCPs is likely to be worthwhile.
1 Pilot program: hand hygiene (HH) compliance at each of the six pilot program hospitals before and after introduction of the HHCCP*
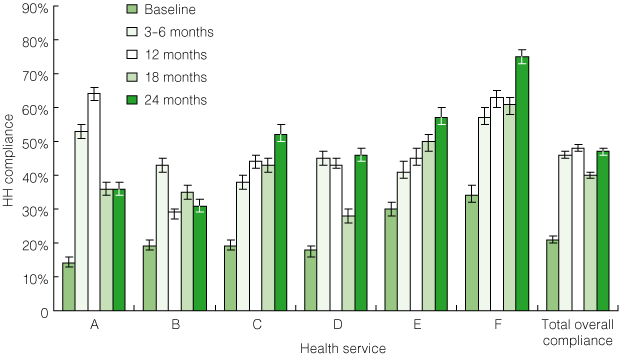 | |||||||||||||||
2 Pilot program: supply of alcohol-based hand-rub solution (ABHRS) on pilot wards before and after introduction of the HHCCP*
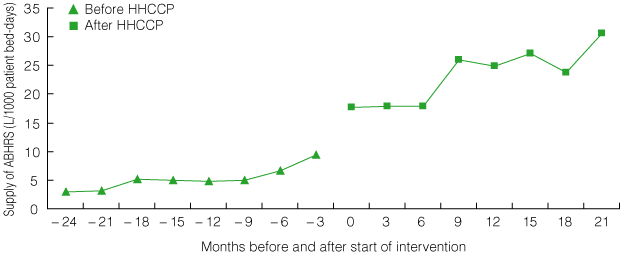 | |||||||||||||||
3 Pilot program: number of patients with MRSA bacteraemia per 100 patient discharges (PD) per month before and after introduction of the HHCCP*
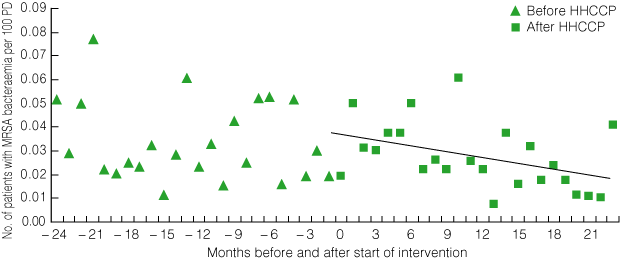 | |||||||||||||||
4 Pilot program: number of clinical MRSA isolates per 100 patient discharges (PD) per month before and after introduction of the HHCCP*
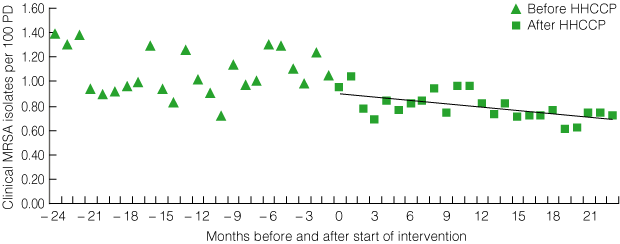 | |||||||||||||||
6 Statewide roll-out: hand hygiene (HH) compliance before and after introduction of the HHCCP, by health service type*
 | |||||||||||||||
7 Statewide roll-out: hand hygiene (HH) compliance before and after introduction of the HHCCP, by stage of roll-out and individual health service*
 | |||||||||||||||
8 Statewide roll-out: patients with MRSA bacteraemia per 100 patient discharges (PD) per month before and after introduction of the HHCCP*
 | |||||||||||||||
9 Statewide roll-out: total clinical MRSA isolates per 100 patient discharges (PD) per month before and after introduction of the HHCCP*
 | |||||||||||||||
Received 21 August 2007, accepted 11 March 2008
- M Lindsay Grayson1,2
- Lisa J Jarvie1
- Rhea Martin1
- Paul D R Johnson1,2
- Meryanda E Jodoin3
- Celene McMullan4
- Roger H C Gregory5
- Kaye Bellis6
- Katie Cunnington7
- Fiona L Wilson8
- Diana Quin9
- Anne-Maree Kelly8,2
- 1 Infectious Diseases and Clinical Epidemiology Department, Austin Health, Melbourne, VIC.
- 2 Department of Medicine, University of Melbourne, Melbourne, VIC.
- 3 Bendigo Health, Bendigo, VIC.
- 4 Melbourne Health, Melbourne, VIC.
- 5 Northeast Health, Wangaratta, VIC.
- 6 Peninsula Health, Melbourne, VIC.
- 7 St Vincent’s Health, Melbourne, VIC.
- 8 Western Health, Melbourne, VIC.
- 9 Victorian Quality Council, Melbourne, VIC.
We would like to thank the following infection control and medical staff for their assistance at various times during the project: Austin Health: Timothy Brown, Laurelle Burrell, Deidre Edmonds; Melbourne Health: David Smallwood; Peninsula Health: Jacqueline Kennon; and Western Health: Jeffery Brooks. We are also grateful to the following key VQC staff, who were important in managing the project at various stages: Robina Bradley, Diana Quin, David Hillis and Jenny Bartlett.
DeBug (a trademark for one of the hand hygiene products referred to in this article) was developed by the authors (employees of Austin Health) with funding in part from the Victorian Department of Human Services. The intellectual property for this development is held by Austin Health, which handles all patent, trademark and licensing issues. Austin Health, but no individual author, receives a small income stream from the sale of DeBug.
- 1. Johnson PDR, Martin R, Burrell LJ, et al. Efficacy of an alcohol/chlorhexidine hand hygiene program in a hospital with high rates of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection. Med J Aust 2005; 183: 509-514. <MJA full text>
- 2. Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet 2000; 356: 1307-1312.
- 3. Grayson ML. The treatment triangle for staphylococcal infections. N Engl J Med 2006; 355: 724-727.
- 4. Wertheim HF, Vos MC, Boelens HA, et al. Low prevalence of methicillin-resistant Staphylococcus aureus (MRSA) at hospital admission in the Netherlands: the value of search and destroy and restrictive antibiotic use. J Hosp Infect 2004; 56: 321-325.
- 5. Pittet D, Boyce JM. Revolutionising hand hygiene in health-care settings: guidelines revisited. Lancet Infect Dis 2003; 3: 269-270.
- 6. World Health Organization. World Alliance for Patient Safety. Global Patient Safety Challenge 2005–2006. Clean care is safer care. Geneva: WHO, 2005. http://www.who.int/patientsafety/events/05/GPSC_Launch_ENGLISH_FINAL.pdf (accessed May 2008).
- 7. Boyce JM, Pittet D; Healthcare Infection Control Practices Advisory Committee; HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Guideline for hand hygiene in health-care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm Rep 2002; 51 (RR-16): 1-45.
- 8. Whitby M, McLaws ML, Ross MW. Why healthcare workers don’t wash their hands: a behavioural explanation. Infect Control Hosp Epidemiol 2006; 27: 484-492.
- 9. Brown TL, Burrell LJ, Edmonds D, et al. Hand-hygiene: a standardised tool for assessing compliance. Aust Infect Control [Internet] 2005; 10: 51-58.
- 10. National Health and Medical Research Council. When does quality assurance in health care require independent ethical review? Canberra: NHMRC, 2003. http://www.nhmrc.gov.au/ethics/human/conduct/guidelines/_files/e46.pdf (accessed May 2008).





Abstract
Objective: To assess the efficacy of a multimodal, centrally coordinated, multisite hand hygiene culture-change program (HHCCP) for reducing rates of methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia and disease in Victorian hospitals.
Design, participants and setting: A pilot HHCCP was conducted over a 24-month period (October 2004 to September 2006) in six Victorian health care institutions (4 urban, 2 rural; total beds, 2379). Subsequently, we assessed the efficacy of an identical program implemented throughout Victorian public hospitals over a 12-month period (beginning between March 2006 and July 2006).
Main outcome measures: Rates of hand hygiene (HH) compliance; rates of MRSA disease (patients with bacteraemia and number of clinical isolates per 100 patient discharges [PD]).
Results: Mean HH compliance improved significantly at all pilot program sites, from 21% (95% CI, 20%–22%) at baseline to 48% (95% CI, 47%–49%) at 12 months and 47% (95% CI, 46%–48%; range, 31%–75%) at 24 months. Mean baseline rates for the number of patients with MRSA bacteraemia and the number of clinical MRSA isolates were 0.05/100 PD per month (range, 0.00–0.13) and 1.39/100 PD per month (range, 0.16–2.39), respectively. These were significantly reduced after 24 months to 0.02/100 PD per month for bacteraemia (P = 0.035 for trend; 65 fewer patients with bacteraemia) and 0.73/100 PD per month for MRSA isolates (P = 0.003; 716 fewer isolates). Similar findings were noted 12 months after the statewide roll-out, with an increase in mean HH compliance (from 20% to 53%; P < 0.001) and reductions in the rates of MRSA isolates (P = 0.043) and bacteraemias (P = 0.09).
Conclusions: Pilot and subsequent statewide implementation of a multimodal HHCCP was effective in significantly improving HH compliance and reducing rates of MRSA infection.