Lung cancer is the commonest cancer among Indigenous Australians. Age-adjusted incidence is about two times higher than for non-Indigenous Australians and age-adjusted mortality is three to four times higher.1-3 Lung cancer accounts for 27% of all cancer deaths among Indigenous Australians, compared with 17% among other Australians.4 Indigenous lung cancer patients in the Northern Territory have been reported to have worse survival than their non-Indigenous counterparts.5 Although reasons for poorer survival have not been established, possibilities include less active treatment, later stage at diagnosis or a higher prevalence of comorbidities such as acute coronary syndrome or chronic bronchitis.
This detailed study of lung cancer among Indigenous patients was nested within a larger, more general study of the diagnosis, treatment and survival of all Indigenous cancer patients compared with non-Indigenous patients treated in Queensland public hospitals.6 Details of the study design have been described fully elsewhere.6
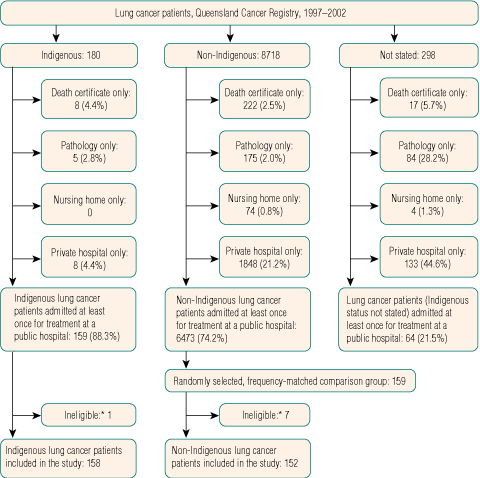
Information on all Indigenous people diagnosed with lung cancer in Queensland for the 6 years 1997–2002 was obtained from the Queensland Cancer Registry. This population-based registry does not collect information on stage at diagnosis, treatment, or comorbidities, so we obtained this information through a review of medical records and linkage to computerised discharge abstracts. Because of legal and administrative difficulties, patients treated exclusively in private hospitals were excluded, as were patients notified only from a nursing home, pathology laboratory, or death certificate (Box 1). Because Indigenous cancer patients tend to be younger and more likely to live in rural or remote areas than non-Indigenous cancer patients,3 we frequency-matched on age and rurality (using the Accessibility/Remoteness Index of Australia),7 as well as on sex, to improve statistical efficiency.8 The medical record from the largest public hospital at which the patient received treatment for lung cancer was reviewed.
Demographic information and all other data from the Queensland Cancer Registry were cross-checked against hospital medical records and updated where necessary. Stage at diagnosis was assigned using coding rules from the Surveillance Epidemiology and End Results program.9 Basic information on cancer treatment, including chemotherapy (yes/no), radiotherapy (yes/no) and surgery (yes/no), was collected. Comorbidities and details of cancer-related surgery were checked against computerised discharge abstracts.
Over the 6-year study period, there were 180 notifications of Indigenous people with lung cancer to the Queensland Cancer Registry. These patients were younger, more likely to live in rural or remote areas, and more likely to be economically disadvantaged than the 8718 non-Indigenous people notified to the registry with lung cancer (Box 2). In contrast, method of diagnosis and subtype were similar between the two groups.
There were 158 Indigenous lung cancer patients eligible for inclusion (Box 1), and when frequency-matched to a random sample of 152 non-Indigenous public-hospital patients, distributions of age, sex, rurality and economic disadvantage were similar in the two groups (Box 3). Of the 158 Indigenous (152 non-Indigenous) lung cancer patients, 137 (122) died during the study period, and for 128 (110), the underlying cause of death was lung cancer.
Method of diagnosis and histological subtype of lung cancer were similar for Indigenous and non-Indigenous patients (Box 4). A larger percentage of Indigenous patients had no information about cancer staging in the chart examined (23% v 13%), and a smaller percentage had localised disease (22% v 30%). Chronic bronchitis or emphysema and diabetes were almost twice as common among the Indigenous cohort, but co-occurrence of heart failure, hypertension and acute coronary syndromes were similar (Box 5). Fewer than six patients in each group had chronic renal failure, liver failure, stroke or dementia.
A smaller percentage of Indigenous than non-Indigenous patients received chemotherapy, radiotherapy or surgery (Box 6). There was a similar pattern for active treatment overall. The treatment disadvantage for the Indigenous cohort remained after adjusting for histological subtype, stage at diagnosis and comorbidities: the adjusted risk ratio comparing the percentage of Indigenous and non-Indigenous patients who received any active treatment was 0.65 (95% CI, 0.53–0.73).
The median lung cancer-specific survival for the Indigenous cohort was 4.3 months (95% CI, 3.1–6.6 months), which was much shorter than for the non-Indigenous patients (10.3 months; 95% CI, 7.1–12.6 months). These disparities were reflected in the unadjusted hazard rate, which was 48% higher among Indigenous than non-Indigenous patients (hazard ratio [HR], 1.48; 95% CI, 1.14–1.92). Because we frequency-matched the study cohorts, the inclusion of age, sex, rurality and economic disadvantage made little difference to the hazard ratio. Similarly, because histological subtype had a similar distribution across both cohorts, it made little difference to the hazard ratio. Although there were more Indigenous patients who were not staged (Box 4), and this was related to worse survival, inclusion of staging in the model did not change the hazard ratio appreciably (HR, 1.50; 95% CI, 1.16–1.96).
Fewer Indigenous patients received treatment with chemotherapy, radiotherapy and surgery (Box 6), and adding these treatment variables to the proportional hazards survival model reduced the hazard ratio to 1.10 (95% CI, 0.83–1.44). Addition of comorbidities (mainly diabetes) further reduced the hazard ratio to 1.02 (95% CI, 0.77–1.35). That is, variation in rates of active treatment accounted for most of the variation in survival, and concurrent chronic diseases accounted for the remaining survival difference between the two groups.
Our previous study of cancer in general showed that Indigenous cancer patients had worse survival than their non-Indigenous counterparts, but this was not explained by disparities in cancer treatment, the higher prevalence of comorbidities, or stage at diagnosis.6 A NT study that examined several cancers combined, including colorectal, breast, cervix and lung cancers, also reported that treatment differences did not explain all the survival disadvantage of Indigenous patients.10
Thus, our specific results for lung cancer survival differ from the results, on average, for several cancers combined. This might be because active treatment is especially critical to survival after a diagnosis of lung cancer, but less so after the diagnosis of other major cancers. For instance, there is good evidence that surgery and radiotherapy for non-small cell lung cancer improves survival (and quality of life), and radiotherapy and chemotherapy optimise survival from small cell lung cancer.11 In our data, numbers were too small for a stratified analysis by subtype, although the subtype distribution was similar in both cohorts and we adjusted for subtype in all the models.
Overseas studies of disadvantaged groups have also found that less active treatment explains disparities in survival for lung cancer.12,13 For example, a study in the United States of patients with potentially resectable non-small cell lung cancer (stage I or II) found that the lower survival of African American patients, compared with white patients, was largely explained by lower rates of surgical treatment.14
Our information on chemotherapy and radiotherapy was limited to whether it was received by the patient, because of difficulties associated with obtaining detailed treatment information retrospectively from medical records. Nevertheless, statistical adjustment using only the basic treatment information available removed almost all the Indigenous survival disadvantage. Also, the low percentage of Indigenous patients having surgery (10%) was the same as that reported in Western Australia.15
We found that 23% of Indigenous patients and 13% of non-Indigenous patients did not have any information in the particular medical record examined about spread of the lung cancer at the time of diagnosis. We did not exclude these patients from our analysis, but statistically adjusted for the “not staged” category as well as “distant”, “regional” and “localised” categories. Moreover, the relatively high percentage of Indigenous patients with no staging information is an important finding in itself. For both small cell and non-small cell lung cancer, outcomes are improved if the patient’s cancer is staged.11
Finally, Indigenous identification in the Queensland Cancer Registry and computerised discharge abstracts is based on self-identification6 (as in all routine datasets in Australia). Because Indigenous status has been routinely collected in Queensland hospitals since 1996, case ascertainment should be reasonably complete. Moreover, we believe our Indigenous to non-Indigenous comparisons to be internally valid with little misclassification of ethnicity, as medical charts were examined for verification.
Other research groups have suggested a link between specialist referral and active treatment of lung cancer. For example, one of the reasons suggested for the lower survival from lung cancer in the United Kingdom compared with other European countries was poorer access to specialised care because of fewer consultants per head of population.16 Similarly, a population-based patterns-of-care study in the United States found that whether patients received chemotherapy for lung cancer was determined by whether they were seen by a specialist.17
In our study, we did not have data on whether patients were referred for a specialist opinion. It would be useful to determine whether Indigenous lung cancer patients are less likely to consult a lung cancer specialist than non-Indigenous patients, and to investigate who makes this decision — the patient or the general practitioner. Also, studies in other settings have suggested that some patients (and their doctors) take an overly fatalistic attitude to lung cancer, which means that the patients receive less than optimal treatment and their quality of life and survival is less than it otherwise might have been.18 This would also be a useful avenue for further investigation.
Indigenous lung cancer patients have particular needs that should be explicitly considered when planning cancer services. For example, they often need to travel long distances to access specialist services, and they may have particularly fatalistic views of lung cancer and its treatment. Although further research is needed, sensible policy initiatives could be implemented immediately given the seriousness of the problem. Initiatives could be evaluated while they are being implemented — an approach that encourages creativity and innovation,18 and would contribute valuable evidence for refining subsequent initiatives.
2 Characteristics of Indigenous and non-Indigenous patients diagnosed with lung cancer, 1997–2002, Queensland
- Michael D Coory1
- Adele C Green2
- Janelle Stirling2
- Patricia C Valery2
- 1 School of Population Health, University of Queensland, Brisbane, QLD.
- 2 Queensland Institute of Medical Research, Brisbane, QLD.
We thank Mrs Valerie Logan for technical support, Ms Judy Symmons for data collection and the Queensland Aboriginal and Islander Health Forum for acting as a community resource to provide community consultation. This work has been produced as part of the activities of the Cooperative Research Centre for Aboriginal Health, a collaborative partnership partly funded by the CRC Programme of the Commonwealth Department of Education, Science and Technology. Perpetual – Derham Green Fund funded the study; Patricia Valery is supported by a National Health and Medical Research Council Public Health (Australia) Training Fellowship.
None identified.
- 1. South Australian Cancer Registry. Cancer incidence, mortality and case survival in the South Australian Aboriginal population. In: Epidemiology of cancer in South Australia 1977 to 1996. Adelaide: South Australian Health Commission, 1997.
- 2. Coory M, Thompson A, Ganguly I. Cancer among people living in rural and remote Indigenous communities. Med J Aust 2000; 173: 301-304.
- 3. Condon J, Armstrong B, Barnes A, Cunningham J. Cancer in Indigenous Australians: a review. Cancer Causes Control 2003; 14: 109-121.
- 4. Australian Bureau of Statistics, Australian Institute of Health and Welfare. The health and welfare of Australia’s Aboriginal and Torres Strait Islander Peoples 2005. Canberra: ABS, 2005. (ABS Cat. No. 4704.0.)
- 5. Condon J, Armstrong B, Barnes T, Zhao Y. Cancer incidence and survival for Indigenous Australians in the Northern Territory. Aust N Z J Public Health 2005; 29: 123-128.
- 6. Valery P, Coory M, Stirling J, Green A. Cancer diagnosis, treatment and survival in Indigenous compared with non-Indigenous Australians treated in public hospitals. Lancet 2006; 367: 1842-1848.
- 7. Australian Government Department of Health and Aged Care. Accessibility/Remoteness Index of Australia. Canberra: DHAC, 1999.
- 8. Rothman K, Greenland S. Modern epidemiology. Philadelphia: Lippincott-Raven, 1998.
- 9. Surveillance Epidemiology and End Results. Summary staging manual 2000: codes and coding instructions. Bethesda: National Cancer Institute, 2001.
- 10. Condon JR, Cunningham J, Barnes T, et al. Cancer diagnosis and treatment in the Northern Territory: assessing health service performance for indigenous Australians. Intern Med J 2006; 36: 498-505.
- 11. National Health and Medical Research Council. Clinical practice guidelines for the prevention, diagnosis and management of lung cancer. Canberra: NHMRC, 2004.
- 12. Greenwald HP, Polissar NL, Borgatta EF, et al. Social factors, treatment, and survival in early-stage non-small cell lung cancer. Am J Public Health 1998; 88: 1681-1684.
- 13. Bach P, Schrag D, Brawley O, et al. Survival of blacks and whites after a cancer diagnosis. JAMA 2002; 287: 2106-2113.
- 14. Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med 1999; 341: 1198-1205.
- 15. Hall S, Bulsara C, Bulsara M, et al. Treatment patterns for cancer in Western Australia: does being Indigenous make a difference? Med J Aust 2004; 181: 191-194. <MJA full text>
- 16. Janssen-Heijnen M, Gatta G, Forman D, et al; Eurocare Working Group. Variation in survival of patients with lung cancer in Europe 1985–1989. Eur J Cancer 1998; 34: 2191-2196.
- 17. Earle C, Neuman P, Gelber R. Impact of referral patterns on the use of chemotherapy for lung cancer. J Clin Oncol 2002; 20: 1786-1792.
- 18. Coote A. Prevention rather than cure. Making the case for choosing health. London: King’s Fund Publications, 2004.





Abstract
Objective: To compare survival of Indigenous and non-Indigenous lung cancer patients and to investigate any corresponding differences in stage, treatment and comorbidities.
Design and setting: Cohort study of 158 Indigenous and 152 non-Indigenous patients (frequency-matched on age, sex and rurality) diagnosed with lung cancer between 1996 and 2002 and treated in Queensland public hospitals.
Main outcome measures: Survival after diagnosis of lung cancer; effects of stage at diagnosis, treatment, comorbidities and histological subtype on lung cancer-specific survival.
Results: Survival of Indigenous lung cancer patients was significantly lower than that of non-Indigenous patients (median survival, 4.3 v 10.3 months; hazard ratio, 1.48; 95% CI, 1.14–1.92). Of 158 Indigenous patients, 72 (46%) received active treatment with chemotherapy, radiotherapy or surgery compared with 109 (72%) of the 152 non-Indigenous patients, and this treatment disparity remained after adjusting for histological subtype, stage at diagnosis, and comorbidities (adjusted risk ratio, 0.65; 95% CI, 0.53–0.73). The treatment disparity explained most of the survival deficit: the hazard ratio reduced to 1.10 (95% CI, 0.83–1.44) after inclusion of treatment variables in the proportional hazards survival model. The remaining survival deficit was explained by the higher prevalence of comorbidities among Indigenous cancer patients, mainly diabetes.
Conclusion: Survival after a diagnosis of lung cancer is worse for Indigenous patients than for non-Indigenous patients, and differences in treatment between the two groups are mainly responsible.