Multidisciplinary care (MDC) is a collaborative approach to cancer care, whereby the treatment plan for the patient is discussed by health professionals in various fields of cancer treatment as an integrated team, considering all treatment options and the preferences of the patient.1,2 MDC has been shown to provide benefits to medical professionals1,3 as well as patients.4,5
The National Health and Medical Research Council (NHMRC) Clinical practice guidelines for the management of early breast cancer state that all patients with early breast cancer should have access to care from a range of disciplines.6
The National Breast Cancer Centre (NBCC) encouraged MDC as a new standard of cancer care, conducting large studies and forums.2,7 The results of these were formulated into guidelines on how to implement MDC.7 Few studies have looked at how these guidelines are followed by the medical community. The definition of MDC remains fluid in the literature and among health professionals, with no one interpretation being a perfect fit for all situations.1,7,8
The National Breast Cancer Audit identified MDC as an important emerging area relating to quality patient care that is not included in the Audit’s dataset. Information on MDC was captured in a questionnaire consisting of 16 items based on the NBCC’s “Principles of multidisciplinary care”.7
The overall response rate was 91.8% (258/281), although 19 of these surgeons replied that they were no longer practising breast surgery, were retired, or were no longer full members of the Section of Breast Surgery, and were excluded from our analysis. The valid survey responses used for analysis therefore gave a response rate of 91.2% (ie, 239/262). The distribution of participating surgeons in public and private practice, and in metropolitan, regional and rural settings is shown in Box 1.
Box 2 shows the establishment of MDC teams by practice type (some surgeons may have both public and private aspects to their practice). While the majority of both public and private practices have MDC teams established, a much higher proportion of public practices have MDC teams.
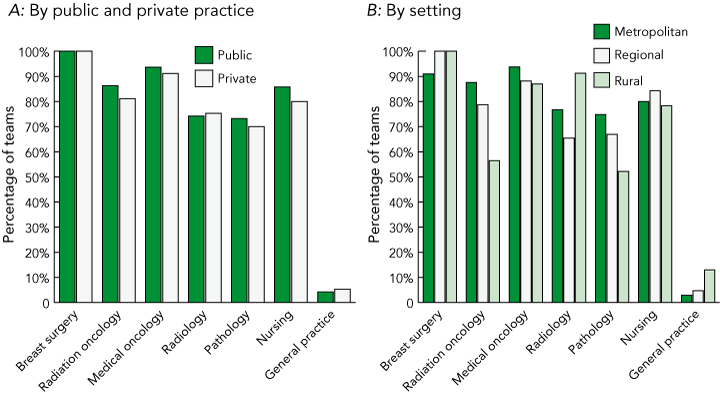
Box 3A shows that the six core specialist disciplines recommended by the NBCC (surgery, medical oncology, radiation oncology, pathology, radiology and supportive care indicated by nursing)2,7 were well represented among MDC teams, and there appears to be little difference between public and private teams. The vast majority of responses categorised as nursing (80.9%) refer to a specialist breast care nurse. Box 3B shows that rural teams were less likely than metropolitan and regional teams to include radiation oncologists and pathologists, possibly because these professionals are in short supply in these areas. Ninety-six MDC teams overall included all six core disciplines.
Very few teams included the patient’s general practitioner as a core or expanded team member (Box 3 and Box 4), despite the recommendation by the NBCC that the GP be included in the treatment team.2,7 Rural teams had the most involvement with GPs.
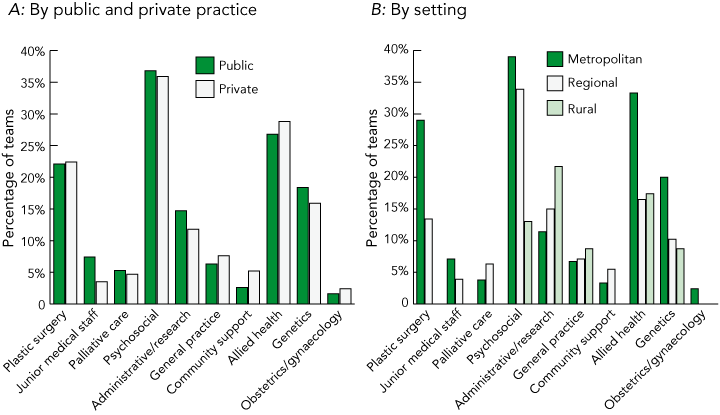
Box 4 shows the teams that included the disciplines shown as members of the expanded MDC team. Most notable additions to the core team were disciplines that focus on the patients’ quality of life and other issues secondary to the cancer — psychosocial, allied health, genetics, and plastic surgery. Box 4B shows that rural teams were lacking these services as part of their expanded team.
Box 5 shows that a weekly meeting was the most popular schedule for MDC teams, although this was significantly less common in private practice. Box 5 also shows that a much higher proportion of private practice MDC collaborations did not fit into a common weekly, fortnightly or monthly schedule, with a significantly higher proportion ticking “other”. In comments about the “other” scheduling category, 24 surgeons (14.1%) noted that their private practice MDC team meetings were not scheduled, but were held on an as-needed, ad-hoc or case-by-case basis. Nine surgeons (4.7%) responded that this was the case for their public practice MDC team. Eight surgeons in private practice (4.6%, compared with three surgeons in public practice [1.6%]) commented that their team did not formally meet at all, but that MDC was coordinated through personal communication between individuals from other disciplines. Rural MDC teams were significantly more likely to have infrequent meetings, either monthly, or at a frequency in the “other” category, suggesting a variable, case-by-case basis (Box 5).
Timing of MDC meeting during treatment: MDC meetings were most commonly held between surgery and adjuvant therapy (Box 5). This was consistent across public and private practice and all settings, although Box 5 suggests that the more geographically remote the practice, the more likely that the timing of the MDC meeting would vary from case to case. Additional surgeon comments indicated that the timing of meetings can depend on the features of individual cases, prescheduled meeting times, or on relapse, recurrence or development of metastases.
Protocols for deciding which patients require discussion: Most public and private MDC teams did not have a local protocol in place for deciding which patients require MDC discussion and prefer to discuss all patients. However, the proportion of MDC teams that used a protocol for choosing patients for discussion was significantly higher among private teams, and the proportion of teams who discuss every patient was higher for public teams (Box 5). Among the MDC teams that did use a protocol for discussing patients, there was little difference between public and private practice in the proportion of patients discussed, with about three-quarters of teams in both sectors discussing more than 50% of patients (71.4% v 74.3%, respectively).
Our results also show that there was a marked difference between the performance of metropolitan and rural MDC teams, with the performance of regional teams falling generally between these. Rural teams were much less likely to have a communication framework in place, and most teams held meetings monthly or on a variable basis. Rural teams were also the most likely to have a protocol in place for selecting which cases would be discussed (rather than routinely discussing all cases). Options to help overcome the tyranny of distance and isolation for small-volume remote surgical practices have been explored.2,7,9 Additional comments from some surgeons indicated that, in areas where MDC is not well established, collaborations with major centres, continuing professional development meetings and use of video and teleconferencing may supplement care where formalised discussion is lacking. However, assuming that the evidence-based MDC guidelines represent the paradigm of best practice for breast cancer care, this raises the question of whether patients treated in some private and rural practices are being offered a lower standard of care.
The NBCC guidelines for MDC recommend that the patient be involved in her treatment plan. One study found that patients’ inclusion in the MDC meeting was generally supported by patients and health professionals.3 While the results of our survey do not suggest that the patient is not involved in decision making, they may reflect surgeons’ attitudes towards the role of patients, perhaps placing them outside the treatment team. There may be a similar attitude to patients’ GPs, given that so few GPs were included in either core or expanded MDC teams. More targeted research could examine the extent to which GPs and patients are being included in MDC meetings, attitudes among health professionals to their inclusion, and barriers to their inclusion.
3 Inclusion of the six recommended specialist disciplines and general practice in the core team by public and private practice and by setting
4 Inclusion of other specialist disciplines and general practice in the expanded team by public and private practice and by setting*
 | |||||||||||||||
Received 6 September 2007, accepted 6 December 2007
- Claire J Marsh1
- Margaret Boult1
- Jim X Wang1
- Guy J Maddern1,2
- David M Roder3
- James Kollias1,4
- 1 Australian Safety and Efficacy Register of New Interventional Procedures – Surgical, Royal Australasian College of Surgeons, Adelaide, SA.
- 2 Adelaide University, Adelaide, SA.
- 3 National Breast and Ovarian Cancer Centre, Sydney, NSW.
- 4 Royal Australasian College of Surgeons Section of Breast Surgery, Adelaide, SA.
We thank the National Breast and Ovarian Cancer Centre, for generously supporting the National Breast Cancer Audit through funding made available by the National Breast Cancer Foundation; the National Breast Cancer Audit Steering Committee (formerly Audit Clinical Advisory Committee) and the former Minimum Standards Subcommittee for their input and endorsement of this survey; and the surgeons who contributed their data.
None identified.
- 1. Zorbas H, Barraclough B, Rainbird K, et al. Multidisciplinary care for women with early breast cancer in the Australian context: what does it mean? Med J Aust 2003; 179: 528-531. <MJA full text>
- 2. National Breast Cancer Centre. Multidisciplinary care in Australia: a national demonstration project in breast cancer. Sydney: NBCC, 2003. http://www.nbcc.org.au/resources/documents/MDS_summaryreport.pdf (accessed Feb 2008).
- 3. Choy ET, Chiu A, Butow P, et al. A pilot study to evaluate the impact of involving breast cancer patients in the multidisciplinary discussion of their disease and treatment plan. Breast 2007; 16: 178-189.
- 4. Sainsbury R, Hayward B, Johnston C, Round C. Influence of clinician workload and patterns of treatment on survival from breast cancer. Lancet 1995; 345: 1265-1270.
- 5. Gabel M, Hilton NE, Nathanson SD. Multidisciplinary breast cancer clinics: do they work. Cancer 1997; 79: 2380-2384.
- 6. National Health and Medical Research Council. Clinical practice guidelines for the management of early breast cancer. Sydney: NHMRC, 1995.
- 7. National Breast Cancer Centre. Making multidisciplinary cancer care a reality: a National Breast Cancer Centre forum series report and recommendations. Sydney: NBCC, 2006. http://www.nbocc.org.au/bestpractice/resources/MDR192_makingmultidisciplin.pdf (accessed Feb 2008).
- 8. Whelan JM, Griffith CDM, Archer T. Breast cancer multi-disciplinary teams in England: much achieved but still more to be done. Breast 2006; 15: 119-122.
- 9. Delaney G, Jacob S, Iedema R, et al. Comparison of face-to-face and videoconferenced multidisciplinary clinical meetings. Australas Radiol 2004; 48: 487-492.





Abstract
Objective: To explore the involvement of members of the Royal Australasian College of Surgeons (RACS) Section of Breast Surgery in Australia and New Zealand in multidisciplinary care (MDC) teams.
Design and setting: Questionnaire sent to all full members of the RACS Section of Breast Surgery in December 2006.
Participants: 239 of 262 active full members of the RACS Section of Breast Surgery (response rate, 91.2%).
Main outcome measures: Surgeons’ use of, and the composition and functioning of, MDC teams in public and private practice, and in metropolitan, regional and rural settings.
Results: 85% of responding surgeons reported participating in at least one fully established MDC team. Public-sector teams were operationally more consistent and functional than private teams, and rural teams were less well developed than those in metropolitan and regional centres. The six core disciplines recommended by the National Breast Cancer Centre appear to be well represented in most teams. Patients and their general practitioners were not considered to be part of the treatment team by surgeons.
Conclusions: MDC is supported by most breast surgeons, but there are deficits in rural areas, and in the private sector relative to the public sector.