It has been known since the mid 19th century that the adrenal cortex is essential for life.1 However, life-saving glucocorticoid replacement therapy for adrenal insufficiency (AI) was not widely available until the clinical introduction of cortisone in 1949.2 The initial standard of care established for glucocorticoid supplementation therapy during stress in hypoadrenal patients was based on early case reports of adrenal crises in patients not receiving adequate perioperative glucocorticoid coverage.3,4 Over the past decade, there has been a shift in clinical practice in favour of giving lower doses and shorter duration of glucocorticoids, according to the severity and duration of illness or surgery.5-7 The recommendations for glucocorticoid supplementation presented here will provide useful information for physicians, anaesthetists, surgeons, dentists, obstetricians and general practitioners. We will not address the controversial issue of relative AI in the setting of critical illness.6,8
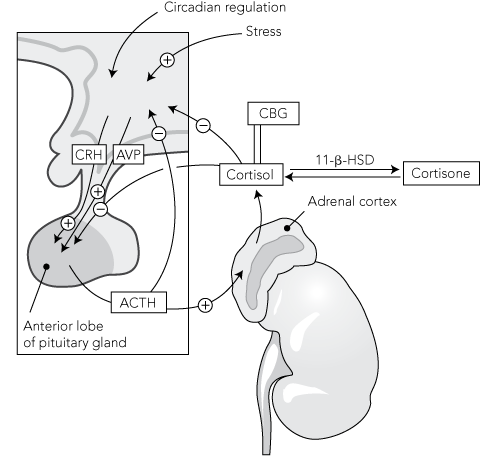
Glucocorticoids are produced in the zona fasciculata of the adrenal cortex under the regulation of the hypothalamic–pituitary–adrenal (HPA) axis (Box 1).7 Corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP), which are synthesised in the hypothalamus, stimulate the secretion of adrenocorticotropic hormone (ACTH) from the pituitary gland, which in turn results in the production of cortisol, the main endogenous glucocorticoid.7,8 Cortisol exerts negative feedback at the level of both the hypothalamus and the pituitary gland.7 Cortisol circulates in the plasma both in the free form (about 5%) and protein-bound (predominantly to corticosteroid-binding globulin [CBG]). Further regulation of glucocorticoids occurs at a cellular level by the action of the 11-β-hydroxysteroid dehydrogenase enzymes and expression of the glucocorticoid receptor.
Earlier estimates of endogenous cortisol production were 12 mg/m2/day.9 However, using newer analytical methods, Esteban et al10 and Kerrigan et al11 showed that the cortisol production rate in normal subjects was significantly lower than previously believed. Both studies found that the mean cortisol production rate was 5.7 mg/m2/day, or about 10 mg/day.10,11
Cortisol has many important metabolic and endocrine functions that are essential for human survival, particularly during stress. Surgery, anaesthesia, trauma, and severe illnesses result in elevated plasma ACTH and cortisol levels.7,8 Cortisol is required for the metabolism of carbohydrates, lipids and proteins, and for the maintenance of vascular tone and endothelial integrity.7,8 It also potentiates the vasoconstrictor actions of catecholamines12 and has anti-inflammatory effects on the immune system.7,8 Aldosterone, synthesised in the adrenal zona glomerulosa under the control of the renin–angiotensin system, regulates sodium and potassium balance and intravascular volume.7
Surgery is one of the most potent activators of the HPA axis.13 Recent studies have reported on HPA axis function during and after various surgical procedures, including cholecystectomy,14 pancreatoduodenectomy,15 coronary artery bypass graft (CABG) surgery,16 pituitary adenomectomy17 and neck exploration.18 The maximum ACTH and cortisol levels are reached in the early postoperative period, especially following anaesthesia reversal and endotracheal extubation.14,16,18 In patients undergoing CABG surgery, plasma cortisol levels increase significantly during the operation, with peak cortisol levels achieved 30 minutes after extubation (median, 744 nmol/L; interquartile range, 645–1062 nmol/L).16 While ACTH levels return to the normal range within 24 hours,8,15-18 cortisol levels decline more slowly, reaching high normal values about 48–72 hours after surgical procedures.8,17,18 From a normal secretion rate of 10 mg/day,10,11 cortisol production rate increases to 75–150 mg/day after major surgery.19
The most common cause of primary AI (Addison’s disease) in developed countries is autoimmune adrenalitis, which can arise in isolation or as part of an autoimmune polyglandular syndrome.20,21 Other causes of primary AI include infections (tuberculosis, cryptococcosis), genetic disorders (adrenoleukodystrophy, adrenomyeloneuropathy), bilateral adrenal haemorrhage, metastases and surgery.20,21 In primary AI, all layers of the adrenal cortex are affected, resulting in glucocorticoid, mineralocorticoid and adrenal androgen deficiencies.20,21
Secondary AI arises from pituitary or hypothalamic dysfunction or failure caused by tumours, irradiation, infiltration, trauma or surgery.20,21 Deficiency of ACTH or CRH leads to atrophy of the adrenal zona fasciculata, resulting in glucocorticoid insufficiency. Mineralocorticoid deficiency does not occur in secondary AI because the renin–angiotensin–aldosterone system remains intact.
Iatrogenic AI is caused by suppression of the HPA axis due to glucocorticoid therapy in pharmacological doses.22 Traditionally, it was believed that the degree of HPA suppression and adrenal atrophy in patients receiving exogenous glucocorticoids was related to the duration and dose of therapy.22,23 In patients taking any steroid dose for less than 3 weeks, suppression of the HPA axis is rarely clinically significant.23 Conversely, any patient who has received the equivalent of 15 mg/day of prednisolone for more than 3 weeks should be suspected of having HPA suppression.23 However, recent studies have found poor correlation between HPA axis function and the cumulative dose, the highest dose or the duration of therapy.24,25 Because of the considerable inter-individual variability in the degree and duration of adrenal suppression, it is difficult to accurately predict which patients will develop AI when glucocorticoid treatment is discontinued.6
Some medications other than glucocorticoids may suppress HPA function and place patients at risk of developing AI. Progestational agents such as medroxyprogesterone and megestrol have glucocorticoid activity.8 Enzyme inducers such as rifampicin and carbamazepine enhance the clearance of some synthetic glucocorticoids. Inhibitors of cortisol synthesis include ketoconazole, aminoglutethimide, and etomidate (an anaesthetic agent that selectively inhibits adrenal 11-β-hydroxylase, the enzyme that converts 11-deoxycortisol to cortisol).26
Glucocorticoid replacement therapy in AI is usually lifelong for patients with adrenal, pituitary or hypothalamic disorders. In patients with iatrogenic AI, glucocorticoid replacement is required until recovery of the HPA axis. Issues regarding the optimal dose, timing of administration and choice of glucocorticoid, as well as methods for assessing replacement therapy, continue to be debated in the literature.20,27
The traditional replacement dosage of hydrocortisone has been 20–30 mg per day, taken orally in divided doses.7 However, based on recent studies of daily cortisol production rates,10,11 some authors have recommended a lower dose of 15 mg daily.27 Optimal hydrocortisone replacement involves dosing two to three times daily,27,28 while longer-acting synthetic glucocorticoids such as prednisolone can be given once daily. The biological half-life and relative potency of commonly used glucocorticoid preparations are outlined in Box 2. Although some experts favour hydrocortisone for replacement therapy in AI,20 there are no outcome data to support the use of one glucocorticoid over another.27 Whatever glucocorticoid is chosen, the clinician must be alert to the clinical features of excessive or inadequate replacement, as no clear optimal method of biochemical monitoring has been established.27 In patients treated with hydrocortisone, use of the cortisol day curve, based on serum samples,28 saliva or blood spots from capillary finger-prick samples,30 has been advocated. Further work is needed to clarify whether such monitoring improves outcomes in patients receiving routine replacement doses.
Patients with primary AI also require mineralocorticoid replacement with fludrocortisone 0.05–0.2 mg daily. The dose is adjusted based on serum sodium and potassium levels, blood pressure and plasma renin activity.7,20,21 Fludrocortisone is not required for treatment of secondary AI. The issue of adrenal androgen replacement with dehydroepiandrosterone20 will not be addressed here.
Soon after the introduction of glucocorticoid therapy for rheumatic diseases in 1949,2 case reports appeared describing perioperative hypotensive crisis and mortality related to presumed iatrogenic AI. In 1952, Fraser et al described a patient with rheumatoid arthritis who developed circulatory shock as a consequence of preoperative withdrawal from glucocorticoid therapy.3 A year later, a similar case report concluded with a list of recommendations for perioperative glucocorticoid coverage (intramuscular cortisone 100 mg daily plus ACTH injections).4
Since then, a number of different schedules for glucocorticoid supplementation therapy have been proposed. These can be broadly divided into two groups. Some have been founded on an empirical basis, often using high-dose glucocorticoid therapy (eg, hydrocortisone 200 mg daily or more for major surgery),13,23,31 while others have been based on the estimated cortisol production rate for different levels of stress.5-7,19,32 Kehlet19 concluded that, based on the available data,33-36 a reasonable estimate of cortisol secretion during the 24 hours after major surgery was 75–150 mg. For major surgery, a hydrocortisone dose of 100 mg/day was recommended; for minor surgery, the recommendation was to give 25 mg hydrocortisone with induction of anaesthesia and the usual glucocorticoid dose postoperatively.
Twenty years later, Salem et al5 reviewed the data on cortisol secretion during surgery and after stimulation with exogenous ACTH, and published recommendations for perioperative glucocorticoid coverage during minor, moderate and major surgical procedures. Inder and Hunt32 proposed a reducing schedule for patients with secondary AI undergoing pituitary surgery: this involved intravenous hydrocortisone 50 mg given 8-hourly on the day of surgery, 25 mg given 8-hourly on the first postoperative day, and a return to maintenance doses by Days 2–3. Subsequent reviews6,7 have also concurred with the rationale for glucocorticoid supplementation that reflects the complexity and duration of the procedures.
In patients with AI who are fasting before procedures, glucocorticoid therapy must be continued, by parenteral routes if necessary. A recent case report has highlighted the adverse consequences of omitting oral steroid therapy in a patient who was fasting before a surgical procedure. The patient developed hypotension and acute renal failure.37 Although it is well known that patients with AI require perioperative glucocorticoid supplementation therapy, serious omissions can occur as a result of inadequate or unclear instructions from the treating team.37 Therefore, it is important to clearly document and institute an appropriate perioperative glucocorticoid management plan.
The findings of studies examining the normal cortisol response to surgery8,17,18 support the concept that increased glucocorticoid coverage is not required in patients with AI beyond 3 days in uncomplicated surgical cases. Potential side effects of prolonged or excessive steroid use include hyperglycaemia, impaired wound healing and an increased susceptibility to infection caused by immune suppression.5,8
Recommendations regarding glucocorticoid coverage during non-surgical illnesses are largely based on expert consensus. Patients have traditionally been advised to double or triple their daily dose of glucocorticoid therapy during a febrile illness until recovery.20,21,27,38 Glucocorticoids should be administered parenterally, preferably intravenously, in cases of vomiting or diarrhoea.20 For patients who have a critical illness such as septic shock, Coursin and Wood7 have recommended 50–100 mg of hydrocortisone every 6–8 hours or 0.18 mg/kg/hr as a continuous intravenous infusion, together with fludrocortisone 0.05 mg daily. The current evidence does not support the use of hydrocortisone doses above 200 mg/day.8 Arafah reported that after intravenous boluses of hydrocortisone 50 mg had been given 6-hourly, peak plasma cortisol levels were over 100 μg/dL (2760 nmol/L), and nadir levels remained elevated at 40–50 μg/dL (1100–1380 nmol/L).8 Keh et al39 showed that, during continuous hydrocortisone infusion (10 mg/hr), plasma total cortisol levels were over 3000 nmol/L — well above the levels reported in patients with septic shock (mean, 880 nmol/L; SEM, 79 nmol/L).40 Another study found that the majority of cortisol levels were between 552 and 1242 nmol/L in intensive care unit patients with severe sepsis or septic shock.41 While it is evident that the glucocorticoid dose should not exceed 200 mg/day,8 the optimal dose for managing septic shock in patients with AI has not been evaluated in controlled clinical trials. Mineralocorticoid supplementation with fludrocortisone is not required in patients with secondary AI or in those with primary AI receiving more than 50 mg hydrocortisone daily, given its potent mineralocorticoid activity at high doses.8
Recommendations for glucocorticoid supplementation therapy during surgery or medical illness are outlined in Box 3. These recommendations are based on extrapolation from what constitutes a normal cortisol response to stress5,19,33-36 and on expert opinion.5-8,20,21,27,32,38 After the administration of intermittent exogenous glucocorticoids, transient increases in plasma cortisol occur that exceed the binding capacity of CBG,42 leading to the rapid clearance of cortisol. Therefore, it may be argued that comparing the total dose of exogenous glucocorticoid required with the endogenous cortisol secretion rate during stress is not valid. However, based on clinical experience and review of the literature, it is safe to treat patients with doses similar to those that mirror the normal physiological response to stress. The recommendations given in Box 3 address the need for increased glucocorticoid supplementation in patients with AI during medical and surgical stress without exposing patients to excessive or prolonged steroid dosing.
During the course of a major illness or surgery, situations may arise in which patients do not appear to “respond” to the recommended glucocorticoid supplementation therapy. It is important to identify and treat other causes of clinical deterioration, such as sepsis or hypovolaemia.6 If there is evidence of a new stressor or a complication, the continued glucocorticoid supplementation should be consistent with the stress response.5
For patients treated with glucocorticoid therapy who are suspected of having iatrogenic AI, some authors have advocated the use of the short ACTH 1-24 (Synacthen) test preoperatively to determine the actual need for increased glucocorticoid supplementation,5 while others have followed the practice of empirical universal coverage given to all patients.22 Providing universal coverage is more feasible than performing preoperative stimulation tests on all such patients.22 Patients receiving topical (inhalation, intranasal or transdermal) glucocorticoids have a low risk of HPA suppression, and some authors have advocated no additional steroid coverage for these patients during minor to moderate illnesses, provided that their clinical course is uncomplicated.6 However, adrenal suppression due to topical glucocorticoids has been described,43 and a case can be made for providing steroid coverage for any patient who has received glucocorticoid therapy for more than 3 weeks by any route. Supplementing these patients, who may be at risk of HPA suppression, eliminates the risk of adrenal crisis. Furthermore, short courses (< 48 hours) of increased glucocorticoid therapy rarely cause significant complications.22
Previous studies have shown that cortisol and ACTH levels increase during normal pregnancy, particularly in the second and third trimesters.44 Some authorities have recommended increasing glucocorticoid replacement doses by 50% in the last trimester of pregnancy for women with AI.20 Whether this is advisable may depend on the patient’s usual treatment dose, as it has been shown that a dose increase is rarely necessary in women treated with 20–30 mg hydrocortisone daily.27,45Steroid management during labour20,27 is outlined in Box 3.
Patients with AI require additional glucocorticoid doses during severe illness or surgery, but the optimal dose, frequency and duration of supplemental therapy remain contentious. Advances in our knowledge of the HPA axis function during stress have prompted reassessment of earlier recommendations for glucocorticoid coverage.5-7 There is no evidence to support excessive dosing (> 200 mg hydrocortisone equivalent/day) or extensive duration of glucocorticoid therapy in uncomplicated cases.8 While these recommendations do not replace clinical judgement, using them will ensure that patients with adrenal insufficiency safely navigate illness episodes or surgical procedures without excessive steroid dosing.
3 Guidelines for glucocorticoid supplementation in patients with adrenal insufficiency
- Caroline Jung1
- Warrick J Inder2
- 1 St Vincent’s Hospital, Melbourne, VIC.
- 2 Department of Medicine, University of Melbourne, Melbourne, VIC.
None identified.
- 1. Addison T. On the constitutional and local effects of diseases of the suprarenal capsules. London: Samuel Highley, 1855.
- 2. Hench PS, Kendall EC, Slocumb CH, et al. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone: compound E) and of pituitary adrenocorticotrophic hormone on rheumatoid arthritis. Proc Staff Meet Mayo Clin 1949; 24: 181-197.
- 3. Fraser CG, Preuss FS, Bigford WD. Adrenal atrophy and irreversible shock associated with cortisone therapy. J Am Med Assoc 1952; 149: 1542-1543.
- 4. Lewis L, Robinson RF, Yee J, et al. Fatal adrenal cortical insufficiency precipitated by surgery during prolonged continuous cortisone treatment. Ann Intern Med 1953; 39: 116-126.
- 5. Salem M, Tainsh RE Jr, Bromberg J, et al. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg 1994; 219: 416-425.
- 6. Lamberts SW, Bruining HA, de Jong FH. Corticosteroid therapy in severe illness. N Engl J Med 1997; 337: 1285-1292.
- 7. Coursin DB, Wood KE. Corticosteroid supplementation for adrenal insufficiency. JAMA 2002; 287: 236-240.
- 8. Arafah BM. Hypothalamic pituitary adrenal function during critical illness: limitations of current assessment methods. J Clin Endocrinol Metab 2006; 91: 3725-3745.
- 9. Kenny FM, Preeyasombat C, Migeon CJ. Cortisol production rate. II. Normal infants, children, and adults. Pediatrics 1966; 37: 34-42.
- 10. Esteban NV, Loughlin T, Yergey AL, et al. Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J Clin Endocrinol Metab 1991; 72: 39-45.
- 11. Kerrigan JR, Veldhuis JD, Leyo SA, et al. Estimation of daily cortisol production and clearance rates in normal pubertal males by deconvolution analysis. J Clin Endocrinol Metab 1993; 76: 1505-1510.
- 12. Udelsman R, Goldstein DS, Loriaux DL, et al. Catecholamine-glucocorticoid interactions during surgical stress. J Surg Res 1987; 43: 539-545.
- 13. Jabbour SA. Steroids and the surgical patient. Med Clin North Am 2001; 85: 1311-1317.
- 14. Donald RA, Perry EG, Wittert GA, et al. The plasma ACTH, AVP, CRH and catecholamine responses to conventional and laparoscopic cholecystectomy. Clin Endocrinol (Oxf) 1993; 38: 609-615.
- 15. Naito Y, Tamai S, Shingu K, et al. Responses of plasma adrenocorticotropic hormone, cortisol, and cytokines during and after upper abdominal surgery. Anesthesiology 1992; 77: 426-431.
- 16. Widmer IE, Puder JJ, König C, et al. Cortisol response in relation to the severity of stress and illness. J Clin Endocrinol Metab 2005; 90: 4579-4586.
- 17. Arafah BM, Kailani SH, Nekl KE, et al. Immediate recovery of pituitary function after transsphenoidal resection of pituitary macroadenomas. J Clin Endocrinol Metab 1994; 79: 348-354.
- 18. Udelsman R, Norton JA, Jelenich SE, et al. Responses of the hypothalamic–pituitary–adrenal and rennin–angiotensin axes and the sympathetic system during controlled surgical and anesthetic stress. J Clin Endocrinol Metab 1987; 64: 986-994.
- 19. Kehlet H. A rational approach to dosage and preparation of parenteral glucocorticoid substitution therapy during surgical procedures. A short review. Acta Anaesthesiol Scand 1975; 19: 260-264.
- 20. Arlt W, Allolio B. Adrenal insufficiency. Lancet 2003; 361: 1881-1893.
- 21. Oelkers W. Adrenal insufficiency. N Engl J Med 1996; 335: 1206-1212.
- 22. Krasner AS. Glucocorticoid-induced adrenal insufficiency. JAMA 1999; 282: 671-676.
- 23. Stewart PM. The adrenal cortex. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams textbook of endocrinology. 10th ed. Philadelphia: Saunders, 2003: 491-551.
- 24. LaRochelle GE Jr, LaRochelle AG, Ratner RE, et al. Recovery of the hypothalamic–pituitary–adrenal (HPA) axis in patients with rheumatic diseases receiving low-dose prednisone. Am J Med 1993; 95: 258-264.
- 25. Schlaghecke R, Kornely E, Santen RT, et al. The effect of long-term glucocorticoid therapy on pituitary-adrenal responses to exogenous corticotropin-releasing hormone. N Engl J Med 1992; 326: 226-230.
- 26. de Jong FH, Mallios C, Jansen C, et al. Etomidate suppresses adrenocortical function by inhibition of 11 beta-hydroxylation. J Clin Endocrinol Metab 1984; 59: 1143-1147.
- 27. Crown A, Lightman S. Why is the management of glucocorticoid deficiency still controversial: a review of the literature. Clin Endocrinol (Oxf) 2005; 63: 483-492.
- 28. Howlett TA. An assessment of optimal hydrocortisone replacement therapy. Clin Endocrinol (Oxf) 1997; 46: 263-268.
- 29. Bahn SL. Glucocorticosteroids in dentistry. J Am Dent Assoc 1982; 105: 476-481.
- 30. Wong V, Yan T, Donald A, et al. Saliva and bloodspot cortisol: novel sampling methods to assess hydrocortisone replacement therapy in hypoadrenal patients. Clin Endocrinol (Oxf) 2004; 61: 131-137.
- 31. Paris J. Pituitary–adrenal suppression after protracted administration of adrenal cortical hormones. Proc Staff Meet Mayo Clin 1961; 36: 305-317.
- 32. Inder WJ, Hunt PJ. Glucocorticoid replacement in pituitary surgery: guidelines for perioperative assessment and management. J Clin Endocrinol Metab 2002; 87: 2745-2750.
- 33. Hardy JD, Turner MD. Hydrocortisone secretion in man: studies of adrenal vein blood. Surgery 1957; 42: 194-201.
- 34. Hume DM, Bell CC, Bartter F. Direct measurement of adrenal secretion during operative trauma and convalescence. Surgery 1962; 52: 174-187.
- 35. Wise L, Margraf HW, Ballinger WF. A new concept on the pre- and postoperative regulation of cortisol secretion. Surgery 1972; 72: 290-299.
- 36. Kehlet H, Binder C. Alterations in distribution volume and biological half-life of cortisol during major surgery. J Clin Endocrinol Metab 1973; 36: 330-333.
- 37. Poulson LK. Acute adrenal insufficiency — withholding of medicines in the peri-operative period. J Pharm Pract Res 2005; 35: 311-312.
- 38. Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med 2003; 348: 727-734.
- 39. Keh D, Boehnke T, Weber-Cartens S, et al. Immunologic and hemodynamic effects of “low-dose” hydrocortisone in septic shock: a double-blind, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med 2003; 167: 512-520.
- 40. Ho JT, Al-Musalhi H, Chapman MJ, et al. Septic shock and sepsis: a comparison of total and free plasma cortisol levels. J Clin Endocrinol Metab 2006; 91: 105-114.
- 41. Sam S, Corbridge TC, Mokhlesi B, et al. Cortisol levels and mortality in severe sepsis. Clin Endocrinol (Oxf) 2004; 60: 29-35.
- 42. Monson JP. The assessment of glucocorticoid replacement therapy. Clin Endocrinol (Oxf) 1997; 46: 269-270.
- 43. Ohman EM, Rogers S, Meenan FO, et al. Adrenal suppression following low-dose topical clobetasol propionate. J R Soc Med 1987; 80: 422-424.
- 44. Carr BR, Parker CR Jr, Madden JD, et al. Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. Am J Obstet Gynecol 1981; 139: 416-422.
- 45. Trainer PJ. Corticosteroids and pregnancy. Semin Reprod Med 2002; 20: 375-380.





Abstract
Patients with adrenal insufficiency (AI) require additional glucocorticoid doses during surgery or medical illness, but there is no universally accepted regimen for glucocorticoid supplementation therapy.
The high doses and long duration of glucocorticoid coverage that have traditionally been used do not reflect the hypothalamic–pituitary–adrenal response to surgical stress and medical illness in normal people.
While the optimal dose and duration of supplementation therapy have not been established, our recommendations are based on extrapolation from what constitutes a normal cortisol response to stress, on expert opinion derived from the medical literature, and on clinical experience.
The recommended use of lower doses of glucocorticoids during surgical and medical stress should not de-emphasise the importance of additional supplementation during such events.
Our recommendations do not replace clinical judgement, but their use will ensure that patients with AI are safely managed during illness or surgery without the risk of an adrenal crisis or excessive steroid dosing.