Monoclonal antibody therapies have become important in many different areas of medicine, from haematological malignancies to inflammatory bowel disease. Infliximab is a chimeric human–mouse immunoglobulin G1 antibody which binds to and blocks tumour necrosis factor α (TNFα), a proinflammatory cytokine. TNFα is thought to play an important role in causing inflammation, which characterises Crohn’s disease. TNFα induces two major signalling cascades: the apoptotic (cell death) pathway, and the inflammatory pathway.1 In Crohn’s disease, infliximab causes apoptosis of T lymphocytes in the lamina propria of the mucosal wall.2
In Crohn’s disease, infliximab is used for patients with moderate to severe mucosal disease refractory to conventional therapy, and for patients with fistulising disease. For patients with mucosal disease, infliximab has been approved for use in Australia under the Pharmaceutical Benefits Scheme beginning in October 2007. For ulcerative colitis, there is evidence for use of infliximab in a severe acute exacerbation refractory to conventional therapy as a means of avoiding surgery,3 and in induction and maintenance therapy in moderate disease.4 Other TNFα inhibitors with trial evidence for use in inflammatory bowel disease include adalimumab5 and certolizumab pegol.6 The soluble TNFα receptor etanercept has not been shown to be efficacious in inflammatory bowel disease, possibly because of less inhibition of lipopolysaccharide-induced interleukin 1β production compared with other TNFα inhibitors.7
Use of TNFα inhibitors is not without risks. A meta-analysis of TNFα inhibitor use in rheumatoid arthritis showed that the risk of serious infection is doubled.8 Reported infections include conventional bacterial infections, such as pneumonia and intravenous line-related sepsis, and opportunistic infections, such as tuberculosis (TB), varicella zoster and histoplasmosis. The effect of TNFα inhibitors on chronic or latent viral infections is less well characterised. Worsening of hepatitis B or hepatitis C infection is possible, and a recent review suggested that all patients should be screened for hepatitis B and C before infliximab therapy.9 Viral reactivation of cytomegalovirus, Epstein–Barr virus or human herpesvirus 6 does not seem to occur.10
Infliximab increases the risk of developing TB fivefold.11 TNFα plays an essential role in host defences against TB through its role in granuloma formation and activation of macrophages.12 Tuberculosis complicating infliximab therapy almost always occurs in patients with pre-existing latent TB infection (LTBI), which is reactivated by the TNFα inhibitor. A third of the world’s population is thought to have LTBI,12 although the proportion is much lower in Australia. Risk factors for LBTI include being born in or having lived in a TB-endemic area, contact with a patient with pulmonary TB, and a prior history of untreated or inadequately treated TB. LTBI is by definition asymptomatic, and only 10% of people with LTBI normally go on to develop active TB.
Treatment of patients for LTBI before TNFα inhibitor use decreases the incidence of active TB by more than 80%.13,14 Traditionally, screening for LTBI has been based on tuberculin (Mantoux) skin testing. However, the Mantoux test has important limitations. False positive results can occur because of BCG vaccination or exposure to environmental (atypical) mycobacteria. False negative results may occur because of immunosuppressive medications or immune deficiency states such as HIV infection. In patients with Crohn’s disease, a false negative Mantoux test may result from either immunosuppressive therapy or from anergy associated with the disease itself.15
New blood tests for detection of TB infection have been introduced recently. These tests are based on measuring the production of interferon γ by T cells in response to in-vitro stimulation by antigens specific to Mycobacterium tuberculosis (and some atypical mycobacteria that are very rare causes of human disease). The QuantiFERON-TB Gold (QFT-G) test (Cellestis, Melbourne, Vic) is an enzyme-linked immunoassay type test that has been endorsed by the United States Centers for Disease Control and Prevention (CDC) for use as an alternative to the Mantoux test for screening selected populations for LTBI. However, the CDC suggest caution in immunosuppressed patients.16 The T-SPOT.TB (Oxford Immunotec, Oxford, UK) is a similar test based on the enzyme-linked immunosorbent spot method.17
Interferon γ release assays (IGRAs) are more convenient and may provide results more quickly than the Mantoux test, and have the advantage of being more specific for M. tuberculosis infection. However, given the lack of a gold standard test for LTBI, determining the true sensitivity of IGRAs is difficult. Studies in patients with culture-proven TB show that IGRAs are at least as sensitive as Mantoux testing.18 Studies in patients at risk of latent TB, such as household contacts of smear-positive pulmonary TB patients, also indicate similar if not superior sensitivity to Mantoux testing, using extent of exposure to the index case as a surrogate marker of likelihood of latent infection.18
Data concerning use of IGRAs in immunosuppressed patients (such as patients on immunomodulator drugs) are still limited. Studies in patients with HIV and in immunosuppressed haematology patients suggest that IGRAs are more sensitive than Mantoux testing for detecting LTBI.19,20 A recent study of patients with inflammatory rheumatic conditions on immunosuppressive medications used risk factors for LTBI as a surrogate marker; this showed a closer association between these risk factors and the IGRA result than with the Mantoux test. Although the Mantoux result was positive more often than the IGRA, this may have represented false positive results due to the high rate of BCG vaccination (83%) in that United Kingdom study, rather than superior sensitivity.21 These immunosuppressed populations may not directly translate to our population of patients with inflammatory bowel disease, but do offer us some information about LTBI screening in the setting of immunosuppression.
Current recommendations for LTBI screening in patients being considered for TNFα inhibitor therapy vary. The British Thoracic Society recommends asking about TB risk factors and performing a chest x-ray, but not a Mantoux test, because of the latter’s reduced sensitivity in the setting of immunosuppression. Treatment of LTBI is considered for selected patients with epidemiological risk factors, even if the chest x-ray is normal (in addition to those with chest x-ray changes or past history of TB).11 The CDC guidelines suggest Mantoux testing of patients with TB risk factors. A chest x-ray is performed only if treatment of LTBI is planned, to exclude active TB. Treatment of LTBI may be considered in patients with a negative Mantoux test if there are convincing epidemiological risk factors.
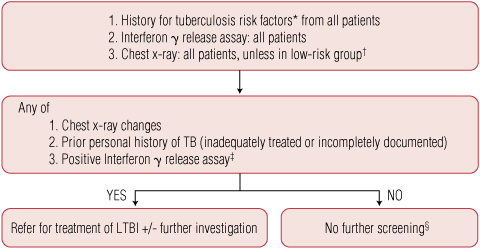
We propose an approach that incorporates an IGRA as an alternative to the Mantoux test (Box). All patients for whom TNFα inhibitor therapy is being considered should have:
an IGRA (such as QFT-G); and
a history taken for TB risk factors and for current symptoms suggestive of active TB; and
a chest x-ray, unless in a low-risk group (Australian-born and younger than 50 years with no other risk factors on history).
positive IGRA;
radiographic lesions suggestive of past TB infection (eg, calcified nodular lesions, apical fibrosis, pleural scarring); or
a history of previous TB (unless an adequate treatment course can be verified).
In some cases, an indeterminate result occurs because of a low or absent positive control response (or alternatively, a high background interferon γ response in the nil control).22 For patients with TB risk factors and an indeterminate result who are not on immunosuppression, the test can be repeated in 2 weeks. If it remains indeterminate (or the patient is on immunosuppression), referral to an infectious diseases or respiratory medicine specialist is advised (Dr David Leslie, Director, Victorian Mycobacterium Reference Laboratory, Victorian Infectious Diseases Reference Laboratory, Melbourne, Vic, personal communication).
If the patient becomes unwell on TNFα inhibitor therapy, the possibility of TB should be considered, even if LTBI treatment has been given or LTBI screening tests are negative. TB often presents with disseminated or extrapulmonary disease in this group of patients, and symptoms may be non-specific and include fever, weight loss and cough.23
In immunosuppressed patients, such as those planned for treatment with TNFα inhibitors, the Mantoux test performs poorly when used for screening for LTBI.11 In these patients, basing decisions about isoniazid treatment of LTBI on clinical risk factors alone runs the risk of over-treatment, with attendant problems such as hepatotoxicity. A screening approach that incorporates an IGRA offers greater specificity and convenience than Mantoux testing. Assessment of the sensitivity of IGRAs is hampered by the lack of a gold standard for diagnosis of LTBI, and further information about sensitivity in immunosuppressed patients is needed. Nevertheless, in addition to their other advantages over Mantoux testing, the improved clinician compliance that should result from use of IGRAs is a strong argument for adopting these tests to screen for LTBI in these patients.
- Arun Gupta1
- Alan C Street2
- Finlay A Macrae3
- Royal Melbourne Hospital, Melbourne, VIC.
None identified.
- 1. Luo JL, Maeda S, Hsu LC, et al. Inhibition of NF-κB in cancer cells converts inflammation-induced tumor growth mediated by TNFα to TRAIL-mediated tumor regression. Cancer Cell 2004; 6: 297-305.
- 2. ten Hove T, van Montfrans C, Peppelenbosch MP, van Deventer SJH. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn’s disease. Gut 2002; 50: 206-211.
- 3. Jarnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 2005; 128: 1805-1811.
- 4. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353: 2462-2476.
- 5. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 2006; 130: 323-333; quiz 591.
- 6. Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med 2007; 357: 228-238.
- 7. Nesbitt A, Fossati G, Bergin M, et al. Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor α agents. Inflamm Bowel Dis 2007; 13: 1323-1332.
- 8. Bongartz T, Sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006; 295: 2275-2285.
- 9. Nathan DM, Angus PW, Gibson PR. Hepatitis B and C virus infections and anti-tumor necrosis factor-α therapy: guidelines for clinical approach. J Gastroenterol Hepatol 2006; 21: 1366-1371.
- 10. Lavagna A, Bergallo M, Daperno M, et al. Infliximab and the risk of latent viruses reactivation in active Crohn’s disease. Inflamm Bowel Dis 2007; 13: 896-902.
- 11. British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-α treatment. Thorax 2005; 60: 800-805.
- 12. Dye C, Scheele S, Dolin P, et al. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 1999; 282: 677-686.
- 13. Perez JL, Kupper H, Spencer-Green GT. Impact of screening for latent TB before initiating anti-TNF therapy in North America and Europe. Ann Rheum Dis 2005; 64 Suppl 3: 265.
- 14. Carmona L, Gomez-Reino JJ, Rodriguez-Valverde V, et al. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum 2005; 52: 1766-1772.
- 15. Mow WS, Abreu-Martin MT, Papadakis KA, et al. High incidence of anergy in inflammatory bowel disease patients limits the usefulness of PPD screening before infliximab therapy. Clin Gastroenterol Hepatol 2004; 2: 309-313.
- 16. Mazurek GH, Jereb J, Lobue P, et al. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep 2005; 54 (RR-15): 49-55.
- 17. Oxford Immunotec [website]. http://www.oxfordimmunotec.com/ (accessed Dec 2007).
- 18. Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146: 340-354.
- 19. Rangaka MX, Diwakar L, Seldon R, et al. Clinical, immunological, and epidemiological importance of antituberculosis T cell responses in HIV-infected Africans. Clin Infect Dis 2007; 44: 1639-1646.
- 20. Piana F, Codecasa LR, Cavallerio P, et al. Use of a T-cell-based test for detection of tuberculosis infection among immunocompromised patients. Eur Respir J 2006; 28: 31-34.
- 21. Matulis G, Jüni P, Villiger PM, Gadola SD. Detection of latent tuberculosis in immunosuppressed patients with autoimmune diseases: performance of a Mycobacterium tuberculosis antigen-specific interferon γ assay. Ann Rheum Dis 2008: 67: 84-90.
- 22. Pai M, Lewinsohn DM. Interferon-γ assays for tuberculosis: is anergy the Achilles’ heel? Am J Respir Crit Care Med 2005; 172: 519-521.
- 23. Winthrop KL. Risk and prevention of tuberculosis and other serious opportunistic infections associated with the inhibition of tumor necrosis factor. Nat Clin Pract Rheumatol 2006; 2: 602-610.





Abstract
Tumour necrosis factor (TNF) α inhibitors such as infliximab are becoming more widely used for the treatment of selected patients with Crohn’s disease, rheumatoid arthritis, and other inflammatory disorders.
TNFα inhibitors increase the risk of serious infections, including tuberculosis.
Screening for and treatment of latent tuberculosis infection before infliximab therapy reduces the risk of developing active tuberculosis.
New blood tests that measure interferon γ production are an alternative to traditional tuberculin skin testing and offer some significant advantages over skin testing for screening of latent tuberculosis infection.