Chlamydia screening, undertaken as part of routine health care for young women, can reduce the incidence of pelvic inflammatory disease and ectopic pregnancy.1 Despite this knowledge, rates of genital chlamydial infection have increased 20%–500% in the past decade in most parts of the developed world.2-4 Chlamydia prevalence is estimated to be at least 1.5%–3.2% in the general population,5,6 and the reported median prevalence in general practice is 4.6% in the United Kingdom,7 1.0%–5.6% in the United States8 and 1.5% in Australia.5
Although there are guidelines for screening in primary care and trials of primary care screening have occurred,9 translating guidelines into practice has been difficult. Barriers to opportunistic chlamydia screening include time pressures, lack of understanding of the benefits of testing and worries about discussing sexual health.10
We have previously proposed that a large proportion of women could be screened for chlamydia if chlamydia testing (CT) occurred in conjunction with the Pap smear11 — a test already firmly established in primary care. Although combining the two tests has been considered in the past,12 and some specialist providers have implemented combined testing (CT/Pap testing) as part of routine care,13 the ability of this approach to increase chlamydia screening has not been formally investigated in general practice. In a preliminary study in general practice, we demonstrated that this method was feasible and acceptable and added only a few minutes to the consultation.11
Our study was conducted between 1 November 2004 and 31 October 2005 in the Australian Capital Territory, which has a population of approximately 325 100.14
As part of the randomisation process, and before consent was obtained from the doctors, eligible general practices were stratified by size (small [1–2 doctors], medium [3–5 doctors] or large [> 5 doctors]) and balanced by an independent statistician to ensure equal numbers between study arms within a stratum. The statistician generated a concealed randomisation schedule for each stratum using a random permuted block design with four practices per block15 and provided the nurse enrolling the practices with three folders (one for each stratum) containing sequentially numbered sealed opaque envelopes. Once at least one doctor in an eligible practice had consented to participate in the study, the nurse opened the envelope and revealed the randomisation status of that general practice.
Practices in the intervention group were asked to offer screening for chlamydia to all eligible women at the same time as performing a Pap smear. Doctors were provided with study packs containing pathology forms and all the equipment needed to collect specimens for Pap and chlamydia testing. They did not receive any other instructions. Control practices were provided with laminated copies of guidelines for chlamydia screening derived from national and international sources7,16-18 (Box 1) and were asked to implement these guidelines in the course of their usual clinical practice.
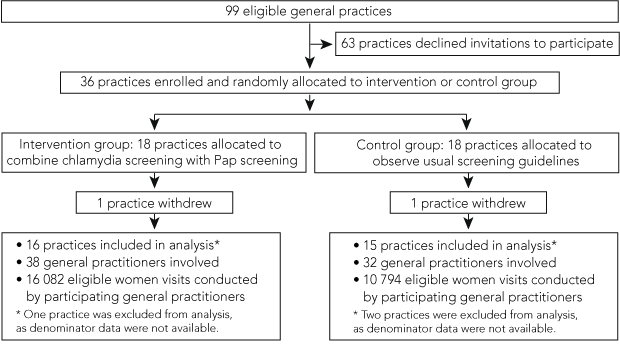
All 99 eligible general practices in the ACT were invited to participate. For the purpose of power calculations we assumed that a third (33) of the general practices would participate in the study. The estimated increase in chlamydia screening rates was based on chlamydia data from local laboratories and a pilot study conducted in the same geographical area.11 There was considerable uncertainty in these estimates because of a lack of information about the number of women attending ACT GPs who would be eligible for our study. We determined that, assuming an intracluster correlation of 0.025,19 33 clusters (general practices) with at least 100 eligible women visits per practice would enable the detection of a quadrupling of effect from 10% to 40% (optimistic outcome) as well as a doubling of effect from 5% to 10% (realistic outcome) with 80% power and a two-sided significance level of 0.05.
Of the 99 eligible general practices approached, 36 (36.4%) were enrolled in the study (18 in each arm). One practice in each arm withdrew during the study period, and denominator data were unobtainable from Medicare Australia for a further three practices (two in the control arm and one in the intervention arm) (Box 2). Characteristics of participating general practices are shown in Box 3.
There were 1590 chlamydia screens performed during the study period (Box 4). Eligible women made 26 876 visits to the participating general practices during the study period — 16 082 visits to intervention practices and 10 794 visits to control practices. Over the 12-month period, chlamydia screening occurred during 6.9% of visits to intervention practices and 4.5% of visits to control practices (Box 5). The chlamydia screening rate per Pap smear among eligible women was 54.1% in intervention practices and 34.8% in control practices (Box 5).
There were no significant differences between the intervention and control arms in the overall rate of positive chlamydia screens or age-specific rates of positive screens per woman screened (Box 6). Neither were there any significant differences in the rate of chlamydia detected by CT/Pap testing (3.5%) or CT alone (4.9%) (Box 6).
After controlling for clustering and potential confounders, there were twofold greater odds of chlamydia screening occurring during a visit by an eligible woman to an intervention practice than to a control practice (adjusted odds ratio, 2.1) (Box 7).
Pap screening (including combined CT/Pap testing) occurred during 12.7% of visits to intervention practices and in 12.9% of visits to control practices (Box 5). Of the 2047 Pap screens carried out during the study, 173 (8.4%) were performed at visits made by women aged < 20 years.
Our study demonstrates that the simple intervention of asking GPs to combine chlamydia screening with Pap screening (without a risk assessment) doubled the odds of chlamydia screening per general practice visit for women aged 16–39 years. Combined CT/Pap screening has been shown to increase chlamydia screening rates in the context of gynaecological and obstetric practice in the US,20 but, to our knowledge, ours is the first study to demonstrate this effect in a primary care setting.
Despite a higher screening rate for chlamydia in our intervention group, the absolute rate of 6.9% per year was still low (although it represents about half the annual Pap smear screening rate in the population studied). The prevalence of chlamydia among tested women was 4.3%, with no statistically significant difference between the rates in the two groups, highlighting the difficulty in identifying those at greater risk of infection. Moreover, there was no statistically significant difference between the 16–19-years age group, the 20–25-years age group and the 25–30-years age group in overall rates of chlamydia infection detected. This suggests that there may be benefit in screening women up to the age of 30 years, even though prevalence and notification data indicate that chlamydia infection rates fall after the age of 25 years.2-4 As the rate in the group aged over 30 years was 0.8%, we would not recommend implementing combined testing in this group.
Randomisation by practice meant that there was variation in the number of participating doctors. Six more doctors were enrolled in the intervention practices, resulting in a higher number of eligible women patients in that group. Although the overall number of male and female GPs in our study was similar, there were significantly fewer male than female doctors in the control practices. Some studies have found that female doctors are more likely to perform Pap smears21,22 and to screen both men and women for STIs, including chlamydia.23,24 It is possible that this sex bias explains some of the difference in the number of chlamydia tests performed in the two arms and the relatively high rate of CT/Pap testing in the control group. The high rate of CT/Pap testing in the control group may also be partly explained by “contamination” of the control group (as the ACT general practice community is relatively small), but also highlights increasing recognition among practitioners of the value of combined testing.
Local ACT and national data indicate that 59%–70% of women aged 16–39 years have a Pap test every 2 years and that, in the 2-year period 2000–2002, 1300 ACT 16–19-year-olds had Pap smears, representing a Pap screening rate in this subpopulation of about 14%.25,26 In light of these figures, the Pap screening rate per visit in our study, although the same for intervention and control groups, was low. This discrepancy may be at least partially explained by the number of Pap smears performed outside general practice (eg, in sexual health and family planning clinics), particularly among women aged 16–39. Adoption of a combined CT/Pap screening approach by all women’s health practitioners, including sexual health and family planning clinics, obstetricians and gynaecologists, is needed to maximise the public health benefit.
Many countries have raised the recommended age of first Pap smear to 25 years.27 If Australia adopted this policy, it would present the ideal opportunity to consider using the existing cervical cancer screening registration and callback infrastructure to implement chlamydia screening for women under 25 years. Women could move from a chlamydia-only screen in their early twenties to a combined CT/Pap screen in their late twenties to a Pap-only screen in their thirties.
1 Targeted chlamydia screening guidelines7,16-18
Offer chlamydia testing to women aged 16–39 years experiencing:
abnormal vaginal discharge;
postcoital or intermenstrual bleeding;
lower abdominal pain;
dyspareunia; or
dysuria
are sexually active and < 25 years of age, or ≥ 25 years of age with a new sexual partner;
have had two or more partners in the past 12 months;
have sexual partner(s) with a sexually transmitted disease;
are seeking uterine instrumentation (eg, termination of pregnancy, intrauterine device insertion; or
are pregnant.
3 Characteristics of participating general practices
Number of practices with at least one female doctor participating |
|||||||||||||||
4 Number of chlamydia and Pap screens performed during the study
CT = chlamydia testing. CT/Pap = combined chlamydia and Pap testing. |
|||||||||||||||
Received 8 July 2007, accepted 14 November 2007
- Francis J Bowden1
- Marian J Currie1
- Helen Toyne1
- Clare McGuiness2
- Lynette L Lim3
- James R Butler4
- Nicholas J Glasgow5
- 1 Academic Unit of Internal Medicine, Australian National University, Canberra, ACT.
- 2 Tuggeranong Square General Practice, Canberra, ACT.
- 3 National Centre for Epidemiology and Population Health, Australian National University, Canberra, ACT.
- 4 Australian Centre for Economic Research on Health, Australian National University, Canberra, ACT.
- 5 Australian Primary Health Care Research Institute, Australian National University, Canberra, ACT.
We wish to thank all the GPs who participated in our study. We also acknowledge the assistance of Sophie Bertram, Leanne Currie and Monique Byrne in managing the project; Sharon Nylen (Capital Pathology), Charmaine Gray and Michelle McNiven (ACT Pathology) and Chris Eldred (Mayne Health Laverty Pathology) in accessing the pathology data; and Medicare Australia for insurance data.
None identified.
- 1. Scholes D, Stergachis A, Heidrich FE, et al. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N Engl J Med 1996; 334: 1362-1366.
- 2. Health Protection Agency Centre for Infections. Diagnoses of selected STIs by region, sex and age group, United Kingdom: 1996–2005. London: HPACI, 2005. http://www.hpa.org.uk/infections//topics_az/hiv_and_sti/epidemiology/2005data/Selected_sti_UK_tables_1996_2005_AR.pdf (accessed Nov 2007).
- 3. Götz H, Lindbäck J, Ripa T, et al. Is the increase in notifications of Chlamydia trachomatis infections in Sweden the result of changes in prevalence, sampling frequency or diagnostic methods? Scand J Infect Dis 2002; 34: 28-34.
- 4. Communicable Diseases Network Australia. National Notifiable Diseases Surveillance System (NNDSS). Canberra: Australian Department of Health and Ageing, Communicable Diseases Control, 2006. http://www.healthconnect.gov.au/internet/wcms/publishing.nsf/Content/cda-surveil-nndss-nndssintro.htm (accessed Nov 2007).
- 5. Vajdic CM, Middleton M, Bowden FJ, et al. The prevalence of genital Chlamydia trachomatis in Australia 1997–2004: a systematic review. Sex Health 2005; 2: 169-183.
- 6. Macleod J, Salisbury C, Low N, et al. Coverage and uptake of systematic postal screening for genital Chlamydia trachomatis and prevalence of infection in the United Kingdom general population: cross sectional study. BMJ 2005; 330: 940-942.
- 7. Chief Medical Officer’s Expert Advisory Group. Main report of the CMO’s Expert Advisory Group on Chlamydia trachomatis. London: Department of Health, 1998.
- 8. Oster NV, Rothenberg R, McPhillips-Tangum CA, et al. Chlamydia screening in a metropolitan Atlanta primary care clinic. South Med J 2003; 96: 863-867.
- 9. Senok A, Wilson P, Reid M, et al. Can we evaluate population screening strategies in UK general practice? A pilot randomised controlled trial comparing postal and opportunistic screening for genital chlamydial infection. J Epidemiol Community Health 2005; 59: 198-204.
- 10. McNulty CA, Freeman E, Bowen J, et al. Barriers to opportunistic chlamydia testing in primary care. Br J Gen Pract 2004; 54: 508-514.
- 11. Toyne H, Glasgow N, McGuiness C, et al. Screening for chlamydia with the Pap test. Aust Fam Physician 2006; 35: 743-744.
- 12. Dorman SA, Danos LM, Wilson DJ, et al. Detection of chlamydial cervicitis by Papanicolaou stained smears and culture. Am J Clin Pathol 1983; 79: 421-425.
- 13. Hawthorne CM, Farber PJ, Bibbo M. Chlamydia/gonorrhea combo and HR HPV DNA testing in liquid-based pap. Diagn Cytopathol 2005; 33: 177-180.
- 14. Australian Bureau of Statistics. Australian demographic statistics. Canberra: ABS, 2004. (ABS Cat. No. 3101.0.)
- 15. Shadbolt B, Wang R, Craft PS. Moving to an on-line framework for knowledge-driven health care. In: Wickramasinghe N, Gupta J, Sharma S, editors. Creating knowledge-based healthcare organizations. Hershey, Pa: Ideas Group, 2004.
- 16. Scottish Intercollegiate Guidelines Network (SIGN). Management of genital Chlamydia trachomatis infection. Edinburgh: Royal College of Physicians, 2000. http://www.sign.ac.uk/pdf/sign42.pdf (accessed Nov 2007).
- 17. National management guidelines for sexually transmissible infections. Melbourne: Venereology Society of Victoria and Australasian College of Sexual Health Physicians, 2002. http://www.mshc.org.au/Portals/6/uploads/National_Management_Guidelines_for_STIs.pdf (accessed Dec 2007).
- 18. Sexually transmitted diseases treatment guidelines 2002. Centers for Disease Control and Prevention. MMWR Recomm Rep 2002; 51 (RR-6): 1-78.
- 19. Campbell MK, Fayers PM, Grimshaw JM. Determinants of the intracluster correlation coefficient in cluster randomized trials: the case of implementation research. Clin Trials 2005; 2: 99-107.
- 20. Burstein GR, Snyder MH, Conley D, et al. Chlamydia screening in a health plan before and after a national performance measure introduction. Obstet Gynecol 2005; 106: 327-334.
- 21. Lurie N, Margolis KL, McGovern PG, et al. Why do patients of female physicians have higher rates of breast and cervical cancer screening? J Gen Intern Med 1997; 12: 34-43.
- 22. Ahmad F, Stewart DE, Cameron JI, Hyman I. Rural physicians’ perspectives on cervical and breast cancer screening: a gender-based analysis. J Womens Health Gend Based Med 2001; 10: 201-208.
- 23. Kufeji O, Slack R, Cassell JA, et al. Who is being tested for genital chlamydia in primary care? Sex Transm Infect 2003; 79: 234-236.
- 24. Freedman E, Britt H, Harrison CM, Mindel A. Sexual health problems managed in Australian general practice: a national, cross sectional survey. Sex Transm Infect 2006; 82: 61-66.
- 25. ACT Cervical Screening Program. Cervical Cytology Register statistical report. Canberra: ACT Health, 2004.
- 26. Australian Institute of Health and Welfare. Cervical screening in Australia 2003–2004. Canberra: AIHW, 2006. (AIHW Cat. No. CAN 28.) http://www.aihw.gov.au/publications/can/csa03-04/csa03-04-c00.pdf (accessed Nov 2007).
- 27. O’Mahony C. First cervical cytology age moved to 25. Int J STD AIDS 2005; 16: 180.





Abstract
Objective: To determine whether asking general practitioners to offer chlamydia screening at the same time as Pap screening increases chlamydia screening rates.
Design: A pragmatic cluster randomised controlled trial.
Participants and setting: Doctors from 31 general practices in the Australian Capital Territory performing more than 15 Pap smear screens per year, and all women aged 16–39 years attending those practitioners between 1 November 2004 and 31 October 2005.
Intervention: Doctors in the intervention practices were asked to routinely offer combined chlamydia and Pap screening to eligible women; doctors in the control practices were asked to implement screening guidelines based on a risk assessment of the individual patient (ie, usual practice).
Main outcome measure: Chlamydia screening rate per visit.
Results: There were 26 876 visits by eligible women during the study period: 16 082 to intervention practices and 10 794 to control practices. Chlamydia screening occurred during 6.9% (95% CI, 6.5%–7.3%) of visits to intervention practices and 4.5% (95% CI, 4.1%–4.9%) of visits to control practices. After controlling for clustering and potential confounders, there were twofold greater odds of chlamydia screening occurring during a visit by an eligible woman to an intervention practice than to a control practice (adjusted odds ratio, 2.1 [95% CI, 1.3–3.4]).
Conclusion: Combining chlamydia and Pap screening increases the rate of chlamydia screening in general practice. Implementing this approach would require little additional infrastructure support in settings where a cervical screening program already exists.