Tako-tsubo cardiomyopathy (TTC) is a recently recognised condition that frequently mimics acute anterior ST-elevation myocardial infarction. All practitioners treating acute chest pain should be aware of TTC. The common form is characterised by acute onset of chest pain, ST elevation in the chest leads, and transient akinesis of the apex and distal half of the anterior, inferior and lateral walls, with compensatory hyperkinesis of the basal walls, which is frequently precipitated by severe psychological or physical stress.1,2 The condition occurs predominantly in postmenopausal women. Left ventricular function recovers rapidly and TTC has an excellent prognosis.
The clinical presentation is almost identical to that of acute anterior myocardial infarction due to coronary occlusion and cannot be reliably diagnosed without coronary angiography. Coronary angiography shows no coronary artery occlusion (or readily identifiable site of probable recent occlusion — a “culprit” lesion) and shows a characteristic wall motion abnormality that cannot be explained by occlusion of a single vessel (ie, involves multiple vascular territories).1 As the wall motion abnormality fully recovers within days to weeks, if angiography is not done early the characteristic left ventricular wall motion abnormality may have resolved and the diagnosis will be missed. This is probably the main reason why the condition has not been widely recognised until recently — with the shift to the use of percutaneous intervention for reperfusion in acute myocardial infarction (rather than thrombolysis), early angiography is now routine in most cases of TTC.
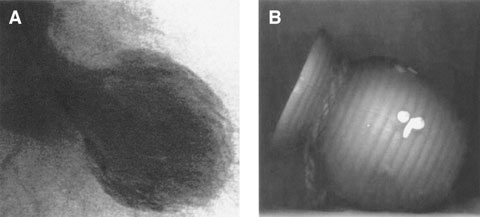
First reported in the Japanese population in the early 1990s,3 the term “tako-tsubo cardiomyopathy” was used to describe this clinical entity because the left ventricle in systole has the shape of a tako-tsubo, a Japanese octopus-catching pot, with a round bottom and a narrow neck (Box 1). It is also commonly known as transient left ventricular apical ballooning syndrome, stress cardiomyopathy4 or broken heart syndrome. None of the names are totally accurate, as more than a third of patients have a small segment of apical sparing,5 and in half to two thirds, TTC is precipitated by physical stress or no stressor is identified.6,7
Although TTC was first recognised more than 15 years ago in Japan, it was not recognised in the Western white population until earlier this decade, and it was thought that its high prevalence in the Japanese population may have been a result of unique genetic or cultural factors. However, more recent reports from North America, Australia, and Eastern Europe, including Asians, African Americans and white people, show that the condition is common worldwide and has been under-recognised.4,7 Indeed, the wide variability in reported prevalence of TTC may simply be due to differences in rates of very early angiography in acute myocardial infarction. We recently reported a TTC rate of 2.6% in patients who presented with ST-elevation acute myocardial infarction to our centre, where all patients have immediate angiography.7 Similarly, a recent review of the literature reported that TTC accounted for about 2% of all patients presenting with chest pain and ST elevation.2
Most patients who present with TTC are postmenopausal women,6 suggesting hormone-related and age-dependent pathophysiology. However, the condition is not exclusively confined to this demographic — there is a higher proportion of premenopausal women among patients with TTC with apical sparing,7 and a higher proportion of men among those with TTC precipitated by physical stress.8
TTC is often referred to as stress cardiomyopathy because the onset of symptoms is frequently preceded by a major stressor — either physical or psychological (Box 2). However, this may be a little misleading: 65%–80% of cases have an identifiable stressor (of which around half are emotional and half physical), but 20%–35% have no obvious precipitant.7
Chest pain is the most common presenting symptom — up to 90% of patients with TTC report chest pain as their primary complaint, although some report only dyspnoea, palpitations, syncope or nausea.4,6,9 As with acute myocardial infarction, features of high circulating adrenaline levels (such as diaphoresis and peripheral shutdown) are also common. In a small proportion (1%–5%), there is significant haemodynamic compromise requiring intra-aortic balloon counterpulsation and mechanical ventilation.6 Other complications are rare; they include left ventricular thrombus formation,10 ventricular rupture11 and intractable arrhythmias.12 In-hospital mortality is < 1%.1
The patient’s electrocardiographic changes on admission are often indistinguishable from those seen in acute anterior myocardial infarction: ST-segment elevation, usually in V3–V6, with evolving T-wave inversion, although ST elevation in other leads and ST depression and T-wave inversion alone have been reported. Some groups have tried to establish criteria to discriminate anterior myocardial infarction from TTC. When the ratio of ST elevation in leads V4–V6 to V1–V3 is > 1.0, this predicts TTC, and the presence of reciprocal inferior ST depression predicts anterior myocardial infarction.13 However, these are not sufficiently accurate to allow confident avoidance of coronary angiography. Later in the course (after 3 days), widespread deep T-wave inversion is often seen with significant QT prolongation, which is not frequently seen after acute myocardial infarction.6 However, these late electrocardiographic discriminators are not sufficiently consistent to allow accurate diagnosis on the basis of electrocardiography alone and do not help to diagnose the patient before angiography.14,15
Serial troponin and creatine kinase MB levels in TTC show only a small rise in comparison to the wall motion abnormality seen; this is an important difference from acute myocardial infarction. A small proportion of patients will have no troponin rise at all, and the absence of elevation does not exclude the diagnosis.7 In addition, the cardiac biomarker levels do not appear to follow the slow rise and fall kinetics seen with myocardial infarctions in which a culprit lesion can be identified.
The diagnosis of TTC depends on coronary angiography. As well as absence of a culprit lesion, there is the characteristic akinesis of the distal half of the anterior and inferior walls and apex on left ventricular angiography (Box 1), which cannot be explained by the occlusion of a single vascular territory (ie, the left anterior descending artery does not extend beyond the apex to supply the distal half of the inferior wall). In the apical sparing variant, this also cannot be explained on the basis of occlusion of a single coronary vessel. The recently published Mayo Clinic criteria1 have been fairly well accepted (Box 3).
It is unusual for serious haemodynamic compromise to persist beyond the time of initial diagnosis. This most frequently occurs when TTC has been precipitated by the stress of another illness. In this circumstance, particularly when the patient requires inotropic support to maintain adequate blood pressure, adrenergic inotropes will only perpetuate the syndrome. In our experience, β-blockade in conjunction with non-adrenergic inotropes can prevent this vicious cycle and allow the ventricle to recover (unpublished data). Particular difficulty may occur when TTC manifests a severe left ventricular outflow tract obstruction (LVOTO), especially when accompanied by severe mitral regurgitation. In one reported case, this required mitral valve replacement.16
The wall motion abnormality in TTC returns to normal in many patients within days,17 and certainly within the first month. This means that TTC patients who do not succumb to haemodynamic compromise have an excellent prognosis with no long-term sequelae. Unless at least echocardiography and preferably coronary angiography are performed within the first 48 hours, the diagnosis may be missed. This would leave the patient with a diagnosis of anterior infarction, with far worse prognosis and resulting undue anxiety.
Recurrence rates are low, with estimates of 5%–10% from long-term follow-up of small to moderate series.6,18 Although α- and β-blocking drugs prevent the syndrome in the rat model,19 and β-blockers prevent the wall motion abnormality when reproduced by dobutamine stress testing in humans,20,21 there have never been any trials for prevention of natural recurrence in the long term. If TTC has been recurrent or the first event has been life-threatening, it seems reasonable to use either β-blockers or combined α-, β-blockade to prevent recurrence. In addition, it seems reasonable to treat people who have significant left ventricular dysfunction with angiotensin-converting enzyme (ACE) inhibitors and β-blockers until ventricular function recovers.
TTC occurs at a time of surging catecholamine levels, which can be precipitated by emotional or physical stress or occur without an identifiable precipitant. Catecholamine levels are characteristically far higher than in matched patients with acute anterior myocardial infarction and similar degrees of left ventricular dysfunction and heart failure.22 On myocardial biopsy, the histological appearances are very similar to contraction band necrosis seen in phaeochromocytoma.22 In a rodent model, TTC can be prevented with α- or β-blockade.19 The more dense distribution of adrenoceptors at the apex might explain why the apex is affected while the base is spared.19 In addition, oestrogen downregulates cardiac adrenoceptors and attenuates their response to activation, providing a plausible reason why the condition is largely confined to postmenopausal women.23
Although there is no dispute that the syndrome is due to a sudden surge in catecholamines, there is considerable controversy as to how this results in the reversible wall motion abnormality. The first and most popular theory attributes the reversible left ventricular dysfunction to microvascular spasm.24 This hypothesis has been supported by observations of increased Thrombolysis In Myocardial Infarction (TIMI) frame counts (ie, slower blood flow) on immediate angiography,25 but this theory does not explain the localisation of the wall motion defect to the distal half of the ventricle, nor why there is apical sparing in some patients. The observation that the transient left ventricular dysfunction is reproducible with dobutamine infusion also does not fit with microvascular spasm as the cause, because dobutamine has only vasodilatory effects.
More recently, it has been proposed that transient severe LVOTO may underlie TTC.26 LVOTO produces ischaemia by markedly increasing oxygen demand and by reducing blood flow by increased wall stress. With severe LVOTO with a concomitant massive catecholamine surge, it is feasible to see myocardial stunning despite normal blood flow. This would be a neat explanation of why the wall motion defect is confined to the distal half of the ventricle, but cannot explain those patients with apical sparing. In addition, older women frequently have sigmoid septum due to unfolding of the aorta, providing an appropriate age- and sex-based predisposition to catecholamine-induced LVOTO. Although a significant gradient develops in some patients and can be reliably reproduced by dobutamine infusion, a gradient was present in only a small proportion of patients at cardiac catheterisation in the largest series reported.6 It is possible that the obstruction is only very transient and has been alleviated by the time of catheterisation. However, some patients have the wall motion abnormality reproduced by dobutamine infusion without any outflow tract gradient.21 It is likely that LVOTO plays a role in exacerbating the syndrome, but does not fully explain it.
Lastly, there is some evidence that TTC may be neurally mediated. Similar transient wall motion abnormalities are seen frequently in patients with subarachnoid haemorrhage, in whom the wall motion abnormality is thought to be due to neurally mediated localised microvascular ischaemia. Histopathological features of the myocardium are very similar, with contraction band necrosis,27 and can be prevented by cardiac sympathectomy.28 Interestingly, apical sparing has also been noted in patients with subarachnoid haemorrhage, and this is associated with an aneurysm in the anterior half of the circle of Willis, although not universally so.29 In this location, aneurysms usually overlie the amygdala and right insular cortex, both of which control sympathetic outflow to the heart providing a mechanistic means to explain the anatomopathological association.29 In support of this hypothesis metaiodobenzylguanidine scanning in the acute phase shows absence of uptake in the affected area.30
1 In tako-tsubo cardiomyopathy, the shape of the left ventricle in systole (A) is very similar to that of a tako-tsubo (a Japanese octopus-catching pot) (B)
3 Mayo Clinic criteria1 for tako-tsubo cardiomyopathy
Transient, reversible akinesis or dyskinesis of the left ventricular apical and mid-ventricular segments with regional wall motion abnormalities extending beyond a single vascular territory on left ventriculography.
Absence of obstructive coronary artery stenosis > 50% of the luminal diameter or angiographic evidence of acute plaque rupture.
New electrocardiographic abnormalities consisting of ST-segment elevation or T-wave inversion.
Absence of:
- Irfan Abdulla1
- Michael R Ward1
- 1 Faculty of Medicine, University of Sydney, Sydney, NSW.
- 2 Department of Cardiology, Royal North Shore Hospital, Sydney, NSW.
None identified.
- 1. Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med 2004; 141: 858-865.
- 2. Gianni M, Dentali F, Grandi AM, et al. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006; 27: 1523-1529.
- 3. Dote K, Sato H, Tateishi H, et al. [Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases] [Japanese]. J Cardiol 1991; 21: 203-214.
- 4. Sharkey SW, Lesser JR, Zenovich AG, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation 2005; 111: 472-479.
- 5. Robles P, Alonso M, Huelmos AI, et al. Images in cardiovascular medicine. Atypical transient left ventricular ballooning without involvement of apical segment. Circulation 2006; 113: e686-e688.
- 6. Tsuchihashi K, Ueshima K, Uchida T, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol 2001; 38: 11-18.
- 7. Abdulla I, Kay S, Mussap C, et al. Apical sparing in tako-tsubo cardiomyopathy. Intern Med J 2006; 36: 414-418.
- 8. Haghi D, Fluechter S, Suselbeck T, et al. Takotsubo cardiomyopathy (acute left ventricular apical ballooning syndrome) occurring in the intensive care unit. Intensive Care Med 2006; 32: 1069-1074.
- 9. Pilliere R, Mansencal N, Digne F, et al. Prevalence of tako-tsubo syndrome in a large urban agglomeration. Am J Cardiol 2006; 98: 662-665.
- 10. Kurisu S, Inoue I, Kawagoe T, et al. Left ventricular apical thrombus formation in a patient with suspected tako-tsubo-like left ventricular dysfunction. Circ J 2003; 67: 556-558.
- 11. Ohara Y, Hiasa Y, Hosokawa S, et al. Left ventricular free wall rupture in transient left ventricular apical ballooning. Circ J 2005; 69: 621-623.
- 12. Konety SH, Horwitz P, Lindower P, Olshansky B. Arrhythmias in tako-tsubo syndrome — benign or malignant? Int J Cardiol 2007; 114: 141-144.
- 13. Ogura R, Hiasa Y, Takahashi T, et al. Specific findings of the standard 12-lead ECG in patients with ‘Takotsubo’ cardiomyopathy: comparison with the findings of acute anterior myocardial infarction. Circ J 2003; 67: 687-690.
- 14. Kurisu S, Inoue I, Kawagoe T, et al. Time course of electrocardiographic changes in patients with tako-tsubo syndrome: comparison with acute myocardial infarction with minimal enzymatic release. Circ J 2004; 68: 77-81.
- 15. Bybee KA, Motiei A, Syed IS, et al. Electrocardiography cannot reliably differentiate transient left ventricular apical ballooning syndrome from anterior ST-segment elevation myocardial infarction. J Electrocardiol 2007; 40: 38.e1-38.e6.
- 16. Chandrasegaram MD, Celermajer DS, Wilson MK. Apical ballooning syndrome complicated by acute severe mitral regurgitation with left ventricular outflow obstruction — case report. J Cardiothorac Surg 2007; 2: 14.
- 17. Kurisu S, Inoue I, Kawagoe T, et al. Documentation of early improvement of left ventricular function in tako-tsubo cardiomyopathy. Int J Cardiol 2007; 114: E70-E72.
- 18. Elesber AA, Prasad A, Lennon RJ, et al. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol 2007; 50: 448-452.
- 19. Ueyama T, Kasamatsu K, Hano T, et al. Emotional stress induces transient left ventricular hypocontraction in the rat via activation of cardiac adrenoceptors: a possible animal model of ‘tako-tsubo’ cardiomyopathy. Circ J 2002; 66: 712-713.
- 20. Brewington SD, Abbas AA, Dixon SR, et al. Reproducible microvascular dysfunction with dobutamine infusion in Takotsubo cardiomyopathy presenting with ST segment elevation. Catheter Cardiovasc Interv 2006; 68: 769-774.
- 21. Kyuma M, Tsuchihashi K, Shinshi Y, et al. Effect of intravenous propranolol on left ventricular apical ballooning without coronary artery stenosis (ampulla cardiomyopathy): three cases. Circ J 2002; 66: 1181-1184.
- 22. Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005; 352: 539-548.
- 23. Ueyama T, Hano T, Kasamatsu K, et al. Estrogen attenuates the emotional stress-induced cardiac responses in the animal model of Tako-tsubo (Ampulla) cardiomyopathy. J Cardiovasc Pharmacol 2003; 42 Suppl 1: S117-S119.
- 24. Kurisu S, Sato H, Kawagoe T, et al. Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction. Am Heart J 2002; 143: 448-455.
- 25. Bybee KA, Prasad A, Barsness GW, et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol 2004; 94: 343-346.
- 26. Merli E, Sutcliffe S, Gori M, Sutherland GG. Tako-Tsubo cardiomyopathy: new insights into the possible underlying pathophysiology. Eur J Echocardiogr 2006; 7: 53-61.
- 27. White M, Wiechmann RJ, Roden RL, et al. Cardiac beta-adrenergic neuroeffector systems in acute myocardial dysfunction related to brain injury. Evidence for catecholamine-mediated myocardial damage. Circulation 1995; 92: 2183-2189.
- 28. Yeh T Jr, Wechsler AS, Graham L, et al. Central sympathetic blockade ameliorates brain death-induced cardiotoxicity and associated changes in myocardial gene expression. J Thorac Cardiovasc Surg 2002; 124: 1087-1098.
- 29. Khush K, Kopelnik A, Tung P, et al. Age and aneurysm position predict patterns of left ventricular dysfunction after subarachnoid hemorrhage. J Am Soc Echocardiogr 2005; 18: 168-174.
- 30. Akashi YJ, Nakazawa K, Sakakibara M, et al. 123 I-MIBG myocardial scintigraphy in patients with “takotsubo” cardiomyopathy. J Nucl Med 2004; 45: 1121-1127.





Abstract
Tako-tsubo cardiomyopathy (TTC) is an important differential diagnosis of acute coronary occlusive myocardial infarction that should be understood by all clinicians.
Although TTC is frequently clinically indistinguishable from acute left anterior descending coronary artery occlusion, it is readily differentiated with coronary angiography. The increasing frequency of acute angiography and revascularisation for patients with acute myocardial infarction has resulted in TTC being far more frequently diagnosed.
Most common in postmenopausal women, TTC is frequently precipitated by physical or emotional stress, and after an acute phase during which the patient may be significantly haemodynamically compromised, there is rapid recovery and an excellent prognosis.
After diagnosis the patient can be reassured and advised of the low rates of recurrence.
Currently, no specific preventive therapy has been proven to be effective.